SYNJARDY Film-coated tablet Ref.[51286] Active ingredients: Empagliflozin Metformin Metformin and Empagliflozin
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Boehringer Ingelheim International GmbH, Binger Str. 173, 55216 Ingelheim am Rhein, Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, combinations of oral blood glucose lowering drugs
ATC code: A10BD20
Mechanism of action
Synjardy combines two antihyperglycaemic medicinal products with complementary mechanisms of action to improve glycaemic control in patients with type 2 diabetes: empagliflozin, an inhibitor of sodium-glucose co-transporter 2 (SGLT2), and metformin hydrochloride, a member of the biguanide class.
Empagliflozin
Empagliflozin is a reversible, highly potent (IC50 of 1.3 nmol) and selective competitive inhibitor of SGLT2. Empagliflozin does not inhibit other glucose transporters important for glucose transport into peripheral tissues and is 5000-times more selective for SGLT2 versus SGLT1, the major transporter responsible for glucose absorption in the gut. SGLT2 is highly expressed in the kidney, whereas expression in other tissues is absent or very low. It is responsible, as the predominant transporter, for the reabsorption of glucose from the glomerular filtrate back into the circulation. In patients with type 2 diabetes and hyperglycaemia a higher amount of glucose is filtered and reabsorbed.
Empagliflozin improves glycaemic control in patients with type 2 diabetes by reducing renal glucose reabsorption. The amount of glucose removed by the kidney through this glucuretic mechanism is dependent on blood glucose concentration and GFR. Inhibition of SGLT2 in patients with type 2 diabetes and hyperglycaemia leads to excess glucose excretion in the urine. In addition, initiation of empagliflozin increases excretion of sodium resulting in osmotic diuresis and reduced intravascular volume.
In patients with type 2 diabetes, urinary glucose excretion increased immediately following the first dose of empagliflozin and is continuous over the 24 hour dosing interval. Increased urinary glucose excretion was maintained at the end of the 4-week treatment period, averaging approximately 78 g/day with empagliflozin 25 mg. Increased urinary glucose excretion resulted in an immediate reduction in plasma glucose levels in patients with type 2 diabetes.
Empagliflozin improves both fasting and post-prandial plasma glucose levels. The mechanism of action of empagliflozin is independent of beta cell function and insulin pathway and this contributes to a low risk of hypoglycaemia. Improvement of surrogate markers of beta cell function including Homeostasis Model Assessment-β (HOMA-β) was noted. In addition, urinary glucose excretion triggers calorie loss, associated with body fat loss and body weight reduction. The glucosuria observed with empagliflozin is accompanied by mild diuresis which may contribute to sustained and moderate reduction of blood pressure. The glucosuria, natriuresis and osmotic diuresis observed with empagliflozin may contribute to the improvement in cardiovascular outcomes.
Metformin
Metformin is a biguanide with antihyperglycaemic effects, lowering both basal and postprandial plasma glucose. It does not stimulate insulin secretion and therefore does not produce hypoglycaemia.
Metformin may act via 3 mechanisms:
- reduction of hepatic glucose production by inhibiting gluconeogenesis and glycogenolysis,
- in muscle, by increasing insulin sensitivity, improving peripheral glucose uptake and utilization,
- and delay of intestinal glucose absorption.
Metformin stimulates intracellular glycogen synthesis by acting on glycogen synthase. Metformin increases the transport capacity of all types of membrane glucose transporters (GLUTs) known to date.
In humans, independently of its action on glycaemia, metformin has favourable effects on lipid metabolism. This has been shown at therapeutic doses in controlled, medium-term or long-term clinical studies: metformin reduces total cholesterol, LDL cholesterol and triglyceride levels.
Clinical efficacy and safety
Both improvement of glycaemic control and reduction of cardiovascular morbidity and mortality are an integral part of the treatment of type 2 diabetes.
Glycaemic efficacy and cardiovascular outcomes have been assessed in a total of 10,366 patients with type 2 diabetes who were treated in 9 double-blind, placebo or active-controlled clinical studies of at least 24 weeks duration, of which 2950 patients received empagliflozin 10 mg and 3701 received empagliflozin 25 mg as add-on to metformin therapy. Of these, 266 or 264 patients were treated with empagliflozin 10 mg or 25 mg as add-on to metformin plus insulin, respectively.
Treatment with empagliflozin in combination with metformin with or without other antidiabetic medicinal products (pioglitazone, sulfonylurea, DPP-4 inhibitors, and insulin) led to clinically relevant improvements in HbA1c, fasting plasma glucose (FPG), body weight, systolic and diastolic blood pressure. Administration of empagliflozin 25 mg resulted in a higher proportion of patients achieving HbA1c goal of less than 7% and fewer patients needing glycaemic rescue compared to empagliflozin 10 mg and placebo. In patients age 75 years and older, numerically lower reductions in HbA1c were observed with empagliflozin treatment. Higher baseline HbA1c was associated with a greater reduction in HbA1c. In addition, empagliflozin as adjunct to standard care therapy reduced cardiovascular mortality in patients with type 2 diabetes and established cardiovascular disease.
Empagliflozin as add-on to metformin, sulphonylurea, pioglitazone
Empagliflozin as add-on to metformin, metformin and a sulphonylurea, or pioglitazone and metformin resulted in statistically significant (p<0.0001) reductions in HbA1c and body weight compared to placebo (Table 3). In addition it resulted in a clinically meaningful reduction in FPG, systolic and diastolic blood pressure compared to placebo.
In the double-blind placebo-controlled extension of these studies, reduction of HbA1c, body weight and blood pressure were sustained up to Week 76.
Table 3. Efficacy results of 24 week placebo-controlled studies:
| Add-on to metformin therapya | |||
| Placebo | Empagliflozin | ||
| 10 mg | 25 mg | ||
| N | 207 | 217 | 213 |
| HbA1c (%) | |||
| Baseline (mean) | 7.90 | 7.94 | 7.86 |
| Change from baseline1 | -0.13 | -0.70 | -0.77 |
| Difference from placebo1 (97.5% CI) | -0.57* (-0.72, -0.42) | -0.64* (-0.79, -0.48) | |
| N | 184 | 199 | 191 |
| Patients () achieving HbA1c <7 with baseline HbA1c ≥7%2 | 12.5 | 37.7 | 38.7 |
| N | 207 | 217 | 213 |
| Body Weight (kg) | |||
| Baseline (mean) | 79.73 | 81.59 | 82.21 |
| Change from baseline1 | -0.45 | -2.08 | -2.46 |
| Difference from placebo1 (97.5% CI) | -1.63* (-2.17, -1.08) | -2.01* (-2.56, -1.46) | |
| N | 207 | 217 | 213 |
| SBP (mmHg)2 | |||
| Baseline (mean) | 128.6 | 129.6 | 130.0 |
| Change from baseline1 | -0.4 | -4.5 | -5.2 |
| Difference from placebo1 (95% CI) | -4.1* (-6.2, -2.1) | -4.8* (-6.9, -2.7) | |
| Add-on to metformin and a sulphonylurea therapya | |||
| Placebo | Empagliflozin | ||
| 10 mg | 25 mg | ||
| N | 225 | 225 | 216 |
| HbA1c (%) | |||
| Baseline (mean) | 8.15 | 8.07 | 8.10 |
| Change from baseline1 | -0.17 | -0.82 | -0.77 |
| Difference from placebo1 (97.5% CI) | -0.64* (-0.79, -0.49) | -0.59* (-0.74, -0.44) | |
| N | 216 | 209 | 202 |
| Patients () achieving HbA1c <7 with baseline HbA1c ≥7%2 | 9.3 | 26.3 | 32.2 |
| N | 225 | 225 | 216 |
| Body Weight (kg) | |||
| Baseline (mean) | 76.23 | 77.08 | 77.50 |
| Change from baseline1 | -0.39 | -2.16 | -2.39 |
| Difference from placebo1 (97.5% CI) | -1.76* (-2.25, -1.28) | -1.99* (-2.48, -1.50) | |
| N | 225 | 225 | 216 |
| SBP (mmHg)2 | |||
| Baseline (mean) | 128.8 | 128.7 | 129.3 |
| Change from baseline1 | -1.4 | -4.1 | -3.5 |
| Difference from placebo1 (95% CI) | -2.7 (-4.6, -0.8) | -2.1 (-4.0, -0.2) | |
| Add-on to pioglitazone + metformin therapyb | |||
| Placebo | Empagliflozin | ||
| 10 mg | 25 mg | ||
| N | 124 | 125 | 127 |
| HbA1c (%) | |||
| Baseline (mean) | 8.15 | 8.07 | 8.10 |
| Change from baseline1 | -0.11 | -0.55 | -0.70 |
| Difference from placebo1 (97.5% CI) | -0.45* (-0.69, -0.21) | -0.60* (-0.83, -0.36) | |
| N | 118 | 116 | 123 |
| Patients () achieving HbA1c <7 with baseline HbA1c ≥7%2 | 8.5 | 22.4 | 28.5 |
| N | 124 | 125 | 127 |
| Body Weight (kg) | |||
| Baseline (mean) | 79.45 | 79.44 | 80.98 |
| Change from baseline1 | 0.40 | -1.74 | -1.59 |
| Difference from placebo1 (97.5% CI) | -2.14* (-2.93, -1.35) | -2.00* (-2.78, -1.21) | |
| N | 124 | 125 | 127 |
| SBP (mmHg)2,3 | |||
| Baseline (mean) | 125.5 | 126.3 | 126.3 |
| Change from baseline1 | 0.8 | -3.5 | -3.3 |
| Difference from placebo1 (95% CI) | -4.2** (-6.94, -1.53) | -4.1** (-6.76, -1.37) | |
a Full analysis set (FAS) using last observation carried forward (LOCF) prior to glycaemic rescue therapy
b Subgroup analysis for patients on additional background of metformin (FAS, LOCF)
1 Mean adjusted for baseline value
2 Not evaluated for statistical significance as a part of the sequential confirmatory testing procedure
3 LOCF, values after antihypertensive rescue censored
* p-value <0.0001
** p-value <0.01
Empagliflozin in combination with metformin in drug-naïve patients
A factorial design study of 24 weeks duration was conducted to evaluate the efficacy and safety of empagliflozin in drug-naïve patients. Treatment with empagliflozin in combination with metformin (5 mg and 500 mg; 5 mg and 1000 mg; 12.5 mg and 500 mg, and 12.5 mg and 1000 mg given twice daily) provided statistically significant improvements in HbA1c (Table 4) and led to greater reductions in FPG (compared to the individual components) and body weight (compared to metformin).
Table 4. Efficacy results at 24 week comparing empagliflozin in combination with metformin to the individual componentsa:
| Empagliflozin 10 mgb | Empagliflozin 25 mgb | Metforminc | ||||||
|---|---|---|---|---|---|---|---|---|
| + Met 1000 mgc | + Met 2000 mgc | No Met | + Met 1000 mgc | + Met 2000 mgc | No Met | 1000 mg | 2000 mg | |
| N | 161 | 167 | 169 | 165 | 169 | 163 | 167 | 162 |
| Baseline (mean) | 8.68 | 8.65 | 8.62 | 8.84 | 8.66 | 8.86 | 8.69 | 8.55 |
| Change from baseline1 | -1.98 | -2.07 | -1.35 | -1.93 | -2.08 | -1.36 | -1.18 | -1.75 |
| Comparison vs. empa (95% CI)1 | -0.63* (-0.86, -0.40) | -0.72* (-0.96, -0.49) | -0.57* (-0.81, -0.34) | -0.72* (-0.95, -0.48) | ||||
| Comparison vs. met (95% CI)1 | -0.79* (-1.03, -0.56) | -0.33* (-0.56, -0.09) | -0.75* (-0.98 -0.51) | -0.33* (-0.56, -0.10) | ||||
Met = metformin; empa = empagliflozin
1 mean adjusted for baseline value
a Analyses were performed on the full analysis set (FAS) using an observed cases (OC) approach
b Given in two equally divided doses per day when given together with metformin
c Given in two equally divided doses per day
* p≤0.0062 for HbA1c
Empagliflozin in patients inadequately controlled with metformin and linagliptin
In patients inadequately controlled with metformin and linagliptin 5 mg, treatment with both empagliflozin 10 mg or 25 mg resulted in statistically significant (p<0.0001) reductions in HbA1c and body weight compared to placebo (Table 5). In addition it resulted in clinically meaningful reductions in FPG, systolic and diastolic blood pressure compared to placebo.
Table 5. Efficacy results of a 24 week placebo-controlled study in patients inadequately controlled with metformin and linagliptin 5 mg:
| Add-on to metformin and linagliptin 5 mg | |||
|---|---|---|---|
| Placebo5 | Empagliflozin6 | ||
| 10 mg | 25 mg | ||
| N | 106 | 109 | 110 |
| HbA1c (%)3 | |||
| Baseline (mean) | 7.96 | 7.97 | 7.97 |
| Change from baseline1 | 0.14 | -0.65 | -0.56 |
| Difference from placebo (95% CI) | -0.79* (-1.02, -0.55) | -0.70* (-0.93, -0.46) | |
| N | 100 | 100 | 107 |
| Patients () achieving HbA1c <7 with baseline HbA1c ≥7%2 | 17.0 | 37.0 | 32.7 |
| N | 106 | 109 | 110 |
| Body Weight (kg)3 | |||
| Baseline (mean) | 82.3 | 88.4 | 84.4 |
| Change from baseline1 | -0.3 | -3.1 | -2.5 |
| Difference from placebo (95% CI) | -2.8* (-3.5, -2.1) | -2.2* (-2.9, -1.5) | |
| N | 106 | 109 | 110 |
| SBP (mmHg)4 | |||
| Baseline (mean) | 130.1 | 130.4 | 131.0 |
| Change from baseline1 | -1.7 | -3.0 | -4.3 |
| Difference from placebo (95% CI) | -1.3 (-4.2, 1.7) | -2.6 (-5.5, 0.4) | |
1 Mean adjusted for baseline value
2 Not evaluated for statistical significance; not part of sequential testing procedure for the secondary endpoints
3 MMRM model on FAS (OC) included baseline HbA1c, baseline eGFR (MDRD), geographical region, visit, treatment, and treatment by visit interaction. For weight, baseline weight was included.
4 MMRM model included baseline SBP and baseline HbA1c as linear covariate(s), and baseline eGFR, geographical region, treatment, visit, and visit by treatment interaction as fixed effects.
5 Patients randomized to the placebo group were receiving the placebo plus linagliptin 5 mg with background metformin
6 Patients randomized to the empagliflozin 10 mg or 25 mg groups were receiving empagliflozin 10 mg or 25 mg and linagliptin 5 mg with background metformin
* p-value <0.0001
In a prespecified subgroup of patients with baseline HbA1c greater or equal than 8.5% the reduction from baseline in HbA1c was -1.3% with empagliflozin 10 mg or 25 mg at 24 weeks (p<0.0001) compared to placebo.
Empagliflozin 24 months data, as add-on to metformin in comparison to glimepiride
In a study comparing the efficacy and safety of empagliflozin 25 mg versus glimepiride (up to 4 mg per day) in patients with inadequate glycaemic control on metformin alone, treatment with empagliflozin daily resulted in superior reduction in HbA1c (Table 6), and a clinically meaningful reduction in FPG, compared to glimepiride. Empagliflozin daily resulted in a statistically significant reduction in body weight, systolic and diastolic blood pressure and a statistically significantly lower proportion of patients with hypoglycaemic events compared to glimepiride (2.5% for empagliflozin, 24.2% for glimepiride, p<0.0001).
Table 6. Efficacy results at 104 week in an active controlled study comparing empagliflozin to glimepiride as add on to metformina:
| Empagliflozin 25 mg | Glimepirideb | |
|---|---|---|
| N | 765 | 780 |
| HbA1c (%) | ||
| Baseline (mean) | 7.92 | 7.92 |
| Change from baseline1 | -0.66 | -0.55 |
| Difference from glimepiride1 (97.5% CI) | -0.11* (-0.20, -0.01) | |
| N | 690 | 715 |
| Patients () achieving HbA1c <7 with baseline HbA1c ≥7%2 | 33.6 | 30.9 |
| N | 765 | 780 |
| Body Weight (kg) | ||
| Baseline (mean) | 82.52 | 83.03 |
| Change from baseline1 | -3.12 | 1.34 |
| Difference from glimepiride1 (97.5% CI) | -4.46** (-4.87, -4.05) | |
| N | 765 | 780 |
| SBP (mmHg)3 | ||
| Baseline (mean) | 133.4 | 133.5 |
| Change from baseline1 | -3.1 | 2.5 |
| Difference from glimepiride1 (97.5% CI) | -5.6** (-7.0,-4.2) | |
a Full analysis set (FAS) using last observation carried forward (LOCF) prior to glycaemic rescue therapy
b Up to 4 mg glimepiride
1 Mean adjusted for baseline value
2 Not evaluated for statistical significance as a part of the sequential confirmatory testing procedure
3 LOCF, values after antihypertensive rescue censored
* p-value <0.0001 for non-inferiority, and p-value = 0.0153 for superiority
** p-value <0.0001
Add-on to insulin therapy
Empagliflozin as add-on to multiple daily insulin
The efficacy and safety of empagliflozin as add-on to multiple daily insulin with concomitant metformin therapy was evaluated in a double-blind, placebo-controlled trial of 52 weeks duration. During the initial 18 weeks and the last 12 weeks, the insulin dose was kept stable, but was adjusted to achieve pre-prandial glucose levels <100 mg/dl [5.5 mmol/l], and post-prandial glucose levels <140 mg/dl [7.8 mmol/l] between Weeks 19 and 40.
At Week 18, empagliflozin provided statistically significant improvement in HbA1c compared with placebo (Table 7).
At Week 52, treatment with empagliflozin resulted in a statistically significant decrease in HbA1c and insulin sparing compared with placebo and a reduction in body weight.
Table 7. Efficacy results at 18 and 52 weeks in a placebo-controlled study of empagliflozin as add-on to multiple daily doses of insulin with concomitant metformin therapy:
| Placebo | empagliflozin | ||
|---|---|---|---|
| 10 mg | 25 mg | ||
| N | 135 | 128 | 137 |
| HbA1c (%) at week 18a | |||
| Baseline (mean) | 8.29 | 8.42 | 8.29 |
| Change from baseline1 | -0.58 | -0.99 | -1.03 |
| Difference from placebo1 (97.5% CI) | -0.41* (-0.61, -0.21) | -0.45* (-0.65, -0.25) | |
| N | 86 | 84 | 87 |
| HbA1c (%) at week 52b | |||
| Baseline (mean) | 8.26 | 8.43 | 8.38 |
| Change from baseline1 | -0.86 | -1.23 | -1.31 |
| Difference from placebo1 (97.5% CI) | -0.37** (-0.67, -0.08) | -0.45* (-0.74, -0.16) | |
| N | 84 | 84 | 87 |
| Patients () achieving HbA1c <7 with baseline HbA1c ≥7% at week 52b,2 | 27.4 | 41.7 | 48.3 |
| N | 86 | 83 | 86 |
| Insulin dose (IU/day) at week 52b,3 | |||
| Baseline (mean) | 91.01 | 91.77 | 90.22 |
| Change from baseline1 | 12.84 | 0.22 | -2.25 |
| Difference from placebo1 (97.5% CI) | -12.61** (-21.43, -3.80) | -15.09** (-23.79, -6.40) | |
| N | 86 | 84 | 87 |
| Body Weight (kg) at week 52b | |||
| Baseline (mean) | 97.78 | 98.86 | 94.93 |
| Change from baseline1 | 0.42 | -2.47 | -1.94 |
| Difference from placebo1 (97.5% CI) | -2.89* (-4.29, -1.49) | -2.37* (-3.75, -0.98) | |
a Subgroup analysis for patients on additional background of metformin (FAS, LOCF)
b Subgroup analysis for patients on additional background of metformin (PPS-Completers, LOCF)
1 Mean adjusted for baseline value
2 not evaluated for statistical significance as a part of the sequential confirmatory testing procedure
3 Week 19-40: treat-to-target regimen for insulin dose adjustment to achieve pre-defined glucose target levels (pre-prandial <100 mg/dl (5.5 mmol/l), post-prandial <140 mg/dl (7.8 mmol/l)
* p-value ≤0.0005
** p-value <0.005
Empagliflozin as add on to basal insulin
The efficacy and safety of empagliflozin as add on to basal insulin with concomitant metformin therapy was evaluated in a double-blind, placebo-controlled trial of 78 weeks duration. During the initial 18 weeks the insulin dose was kept stable, but was adjusted to achieve a FPG <110 mg/dl in the following 60 weeks.
At week 18, empagliflozin provided statistically significant improvement in HbA1c. A greater proportion of patients treated with empagliflozin and with a baseline HbA1c ≥7.0% achieved a target HbA1c of <7% compared to placebo (Table 8).
At 78 weeks, the decrease in HbA1c and insulin sparing effect of empagliflozin was maintained.
Furthermore, empagliflozin resulted in a reduction in FPG, body weight and blood pressure.
Table 8. Efficacy results at 18 and 78 weeks in a placebo-controlled study of empagliflozin as add on to basal insulin with metformina:
| Placebo | Empagliflozin 10 mg | Empagliflozin 25 mg | |
|---|---|---|---|
| N | 96 | 107 | 99 |
| HbA1c (%) at week 18 | |||
| Baseline (mean) | 8.02 | 8.21 | 8.35 |
| Change from baseline1 | -0.09 | -0.62 | -0.72 |
| Difference from placebo1 (97.5% CI) | -0.54* (-0.77, -0.30) | -0.63* (-0.88, -0.39) | |
| N | 89 | 105 | 94 |
| HbA1c (%) at week 78 | |||
| Baseline (mean) | 8.03 | 8.24 | 8.29 |
| Change from baseline1 | -0.08 | -0.42 | -0.71 |
| Difference from placebo1 (97.5% CI) | -0.34** (-0.64, -0.05) | -0.63* (-0.93, -0.33) | |
| N | 89 | 105 | 94 |
| Basal insulin dose (IU/day) at week 78 | |||
| Baseline (mean) | 49.61 | 47.25 | 49.37 |
| Change from baseline1 | 4.14 | -2.07 | -0.28 |
| Difference from placebo1 (97.5% CI) | -6.21** (-11.81, -0.61) | -4.42 (-10.18, 1.34) | |
a Subgroup analysis of full analysis set (FAS) for patients on additional background of metformin – Completers using last observation carried forward (LOCF) prior to glycaemic rescue therapy
1 mean adjusted for baseline value
* p-value <0.0001
** p-value ≤0.025
Empagliflozin and linagliptin as add-on therapy to metformin
In a double-blind trial in patients with inadequate glycemic control, 24-weeks treatment with both doses of empagliflozin plus linagliptin as add-on to metformin therapy provided statistically significant (p<0.0001) reductions in HbA1c (change from baseline of -1.08% for empagliflozin 10 mg plus linagliptin 5 mg, -1.19% for empagliflozin 25 mg plus linagliptin 5 mg, -0.70% for linagliptin 5 mg). Compared to linagliptin 5 mg, both doses of empagliflozin plus linagliptin 5 mg provided statistically significant reductions in FPG and blood pressure. Both doses provided similar statistically significant reductions in body weight, expressed as kg and percentage change. A greater proportion of patients with a baseline HbA1c ≥7.0% and treated with empagliflozin plus linagliptin achieved a target HbA1c of <7% compared to linagliptin 5 mg. Clinically meaningful reductions in HbA1c were maintained for 52 weeks.
Empagliflozin twice daily versus once daily as add on to metformin therapy
The efficacy and safety of empagliflozin twice daily versus once daily (daily dose of 10 mg and 25 mg) as add-on therapy in patients with in sufficient glycemic control on metformin monotherapy was evaluated in a double blind placebo-controlled study of 16 weeks duration. All treatments with empagliflozin resulted in significant reductions in HbA1c from baseline (total mean 7.8%) after 16 weeks of treatment compared with placebo. Empagliflozin twice daily dose regimens on a background of metformin led to comparable reductions in HbA1c versus once daily dose regimens with a treatment difference in HbA1c reductions from baseline to week 16 of -0.02% (95% CI -0.16, 0.13) for empagliflozin 5 mg twice daily versus 10 mg once daily, and -0.11% (95% CI -0.26, 0.03) for empagliflozin 12.5 mg twice daily versus 25 mg once daily.
Cardiovascular outcome
The double-blind, placebo-controlled EMPA-REG OUTCOME study compared pooled doses of empagliflozin 10 mg and 25 mg with placebo as adjunct to standard care therapy in patients with type 2 diabetes and established cardiovascular disease. A total of 7020 patients were treated (empagliflozin 10 mg: 2345, empagliflozin 25 mg: 2342, placebo: 2333) and followed for a median of 3.1 years. The mean age was 63 years, the mean HbA1c was 8.1%, and 71.5% were male. At baseline, 74% of patients were being treated with metformin, 48% with insulin, and 43% with a sulfonylurea. About half of the patients (52.2%) had an eGFR of 60-90 ml/min/1.73 m², 17.8% of 45-60 ml/min/1.73 m² and 7.7% of 30-45 ml/min/1.73 m².
At week 12, an adjusted mean (SE) improvement in HbA1c when compared to baseline of 0.11% (0.02) in the placebo group, 0.65% (0.02) and 0.71% (0.02) in the empagliflozin 10 and 25 mg groups was observed. After the first 12 weeks glycaemic control was optimized independent of investigative treatment. Therefore the effect was attenuated at week 94, with an adjusted mean (SE) improvement in HbA1c of 0.08% (0.02) in the placebo group, 0.50% (0.02) and 0.55% (0.02) in the empagliflozin 10 and 25 mg groups.
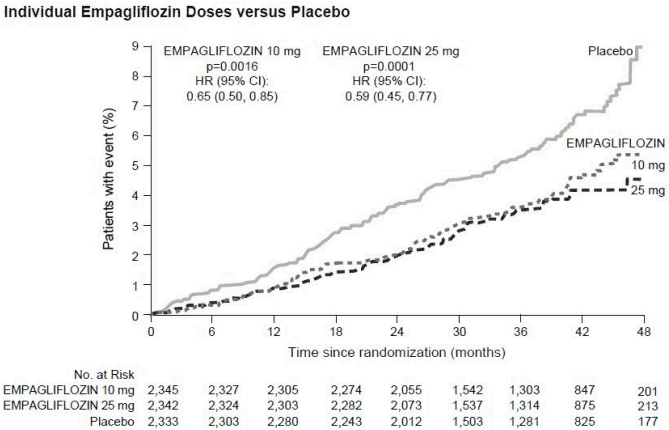
Empagliflozin was superior in preventing the primary combined endpoint of cardiovascular death, non-fatal myocardial infarction, or non-fatal stroke, as compared with placebo. The treatment effect was driven by a significant reduction in cardiovascular death with no significant change in non-fatal myocardial infarction, or non-fatal stroke. The reduction of cardiovascular death was comparable for empagliflozin 10 mg and 25 mg (see Figure 1) and confirmed by an improved overall survival (Table 9). The effect of empagliflozin on the primary combined endpoint of CV death, non-fatal MI, or nonfatal stroke was largely independent of glycaemic control or renal function (eGFR) and generally consistent across eGFR categories down to an eGFR of 30 ml/min/1.73 m² in the EMPA-REG OUTCOME study.
The efficacy for preventing cardiovascular mortality has not been conclusively established in patients using empagliflozin concomitantly with DPP-4 inhibitors or in Black patients because the representation of these groups in the EMPA-REG OUTCOME study was limited.
Table 9. Treatment effect for the primary composite endpoint, its components and mortalitya:
| Placebo | Empagliflozinb | |
|---|---|---|
| N | 2333 | 4687 |
| Time to first event of CV death, non-fatal MI, or non-fatal stroke N (%) | 282 (12.1) | 490 (10.5) |
| Hazard ratio vs. placebo (95.02% CI)* | 0.86 (0.74, 0.99) | |
| p−value for superiority | 0.0382 | |
| CV Death N (%) | 137 (5.9) | 172 (3.7) |
| Hazard ratio vs. placebo (95% CI) | 0.62 (0.49, 0.77) | |
| p-value | <0.0001 | |
| Non-fatal MI N (%) | 121 (5.2) | 213 (4.5) |
| Hazard ratio vs. placebo (95% CI) | 0.87 (0.70, 1.09) | |
| p−value | 0.2189 | |
| Non-fatal stroke N (%) | 60 (2.6) | 150 (3.2) |
| Hazard ratio vs. placebo (95% CI) | 1.24 (0.92, 1.67) | |
| p−value | 0.1638 | |
| All-cause mortality N (%) | 194 (8.3) | 269 (5.7) |
| Hazard ratio vs. placebo (95% CI) | 0.68 (0.57, 0.82) | |
| p-value | <0.0001 | |
| Non-CV mortality N (%) | 57 (2.4) | 97 (2.1) |
| Hazard ratio vs. placebo (95% CI) | 0.84 (0.60, 1.16) |
CV = cardiovascular, MI = myocardial infarction
a Treated set (TS), i.e. patients who had received at least one dose of study drug
b Pooled doses of empagliflozin 10 mg and 25 mg
* Since data from the trial were included in an interim analysis, a two-sided 95.02% confidence interval applied which corresponds to a p-value of less than 0.0498 for significance.
Figure 1. Time to occurrence of cardiovascular death in the EMPA-REG OUTCOME study:
Heart failure requiring hospitalization
In the EMPA-REG OUTCOME study, empagliflozin reduced the risk of heart failure requiring hospitalization compared with placebo (empagliflozin 2.7%; placebo 4.1%; HR 0.65, 95% CI 0.50, 0.85).
Nephropathy
In the EMPA-REG OUTCOME study, for time to first nephropathy event, the HR was 0.61 (95% CI 0.53, 0.70) for empagliflozin (12.7%) vs placebo (18.8%). In addition, empagliflozin showed a higher (HR 1.82, 95% CI 1.40, 2.37) occurrence of sustained normo- or micro-albuminuria (49.7%) in patients with baseline macro-albuminuria compared with placebo (28.8%).
2 hour post-prandial glucose
Treatment with empagliflozin as add-on to metformin or metformin plus sulfonylurea resulted in clinically meaningful improvement of 2-hour post-prandial glucose (meal tolerance test) at 24 weeks (add-on to metformin, placebo: +5.9 mg/dl, empagliflozin 10 mg: -46.0 mg/dl, empagliflozin 25 mg: -44.6 mg/dl; add-on to metformin plus sulphonylurea, placebo: -2.3 mg/dl, empagliflozin 10 mg: -35.7 mg/dl, empagliflozin 25 mg: -36.6 mg/dl).
Patients with baseline HbA1c ≥9%
In a pre-specified analysis of subjects with baseline HbA1c ≥9.0%, treatment with empagliflozin 10 mg or 25 mg as add-on to metformin resulted in statistically significant reductions in HbA1c at Week 24 (adjusted mean change from baseline of -1.49% for empagliflozin 25 mg, -1.40% for empagliflozin 10 mg, and -0.44% for placebo).
Body weight
In a pre-specified pooled analysis of 4 placebo controlled studies, treatment with empagliflozin (68% of all patients were on metformin background) resulted in body weight reduction compared to placebo at week 24 (-2.04 kg for empagliflozin 10 mg, -2.26 kg for empagliflozin 25 mg and -0.24 kg for placebo) that was maintained up to week 52 (-1.96 kg for empagliflozin 10 mg, -2.25 kg for empagliflozin 25 mg and -0.16 kg for placebo).
Blood pressure
The efficacy and safety of empagliflozin was evaluated in a double-blind, placebo controlled study of 12 weeks duration in patients with type 2 diabetes and high blood pressure on different antidiabetic and up to 2 antihypertensive therapies. Treatment with empagliflozin once daily resulted in statistically significant improvement in HbA1c, and 24 hour mean systolic and diastolic blood pressure as determined by ambulatory blood pressure monitoring (Table 10). Treatment with empagliflozin provided reductions in seated SBP and DBP.
Table 10. Efficacy results at 12 week in a placebo-controlled study of empagliflozin in patients with type 2 diabetes and uncontrolled blood pressurea:
| Placebo | empagliflozin | ||
|---|---|---|---|
| 10 mg | 25 mg | ||
| N | 271 | 276 | 276 |
| HbA1c (%) at week 121 | |||
| Baseline (mean) | 7.90 | 7.87 | 7.92 |
| Change from baseline2 | 0.03 | -0.59 | -0.62 |
| Difference from placebo1 (95% CI)2 | -0.62* (-0.72, -0.52) | -0.65* (-0.75, -0.55) | |
| 24 hour SBP at week 123 | |||
| Baseline (mean) | 131.72 | 131.34 | 131.18 |
| Change from baseline4 | 0.48 | -2.95 | -3.68 |
| Difference from placebo4 (95% CI) | -3.44* (-4.78, -2.09) | -4.16* (-5.50, -2.83) | |
| 24 hour DBP at week 123 | |||
| Baseline (mean) | 75.16 | 75.13 | 74.64 |
| Change from baseline5 | 0.32 | -1.04 | -1.40 |
| Difference from placebo5 (95% CI) | -1.36** (-2.15, -0.56) | -1.72* (-2.51, -0.93) | |
a Full analysis set (FAS)
1 LOCF, values after taking antidiabetic rescue therapy censored
2 Mean adjusted for baseline HbA1c, baseline eGFR, geographical region and number of antihypertensive medicinal products
3 LOCF, values after taking antidiabetic rescue therapy or changing antihypertensive rescue therapy censored
4 Mean adjusted for baseline SBP, baseline HbA1c, baseline eGFR, geographical region and number of antihypertensive medicinal products
5 Mean adjusted for baseline DBP, baseline HbA1c, baseline eGFR, geographical region and number of antihypertensive medicinal products
* p-value <0.0001
** p-value <0.001
In a pre-specified pooled analysis of 4 placebo-controlled studies, treatment with empagliflozin (68% of all patients were on metformin background) resulted in a reduction in systolic blood pressure (empagliflozin 10 mg: -3.9 mmHg, empagliflozin 25 mg: -4.3 mmHg) compared with placebo (-0.5 mmHg), and in diastolic blood pressure (empagliflozin 10 mg: -1.8 mmHg, empagliflozin 25 mg: -2.0 mmHg) compared with placebo (-0.5 mmHg), at week 24, that were maintained up to week 52.
Metformin
The prospective randomised (UKPDS) study has established the long-term benefit of intensive blood glucose control in type 2 diabetes. Analysis of the results for overweight patients treated with metformin after failure of diet alone showed:
- a significant reduction of the absolute risk of any diabetes-related complication in the metformin group (29.8 events/1,000 patient-years) versus diet alone (43.3 events/1,000 patient-years), p=0.0023, and versus the combined sulphonylurea and insulin monotherapy groups (40.1 events/1,000 patient-years), p=0.0034,
- a significant reduction of the absolute risk of any diabetes-related mortality: metformin 7.5 events/1,000 patient-years, diet alone 12.7 events/1,000 patient-years, p=0.017,
- a significant reduction of the absolute risk of overall mortality: metformin 13.5 events/1,000 patient-years versus diet alone 20.6 events/1,000 patient-years, (p=0.011), and versus the combined sulphonylurea and insulin monotherapy groups 18.9 events/1,000 patient-years (p=0.021),
- a significant reduction in the absolute risk of myocardial infarction: metformin 11 events/1,000 patient-years, diet alone 18 events/1,000 patient-years, (p=0.01).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Synjardy in all subsets of the paediatric population in type 2 diabetes (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Synjardy
The results of bioequivalence studies in healthy subjects demonstrated that Synjardy (empagliflozin/metformin hydrochloride) 5 mg/850 mg, 5 mg/1,000 mg, 12.5 mg/850 mg, and 12.5 mg/1,000 mg combination tablets are bioequivalent to co-administration of corresponding doses of empagliflozin and metformin as individual tablets.
Administration of empagliflozin/metformin 12.5 mg/1,000 mg under fed conditions resulted in 9% decrease in AUC and a 28% decrease in Cmax for empagliflozin, when compared to fasted conditions. For metformin, AUC decreased by 12% and Cmax decreased by 26% compared to fasting conditions. The observed effect of food on empagliflozin and metformin is not considered to be clinically relevant. However, as metformin is recommended to be given with meals, Synjardy is also proposed to be given with food.
The following statements reflect the pharmacokinetic properties of the individual active substances of Synjardy.
Empagliflozin
Absorption
The pharmacokinetics of empagliflozin have been extensively characterised in healthy volunteers and patients with type 2 diabetes. After oral administration, empagliflozin was rapidly absorbed with peak plasma concentrations occurring at a median tmax of 1.5 hours post-dose. Thereafter, plasma concentrations declined in a biphasic manner with a rapid distribution phase and a relatively slow terminal phase. The steady state mean plasma AUC and Cmax were 1870 nmol.h/l and 259 nmol/l with empagliflozin 10 mg and 4740 nmol.h/l and 687 nmol/l with empagliflozin 25 mg once daily. Systemic exposure of empagliflozin increased in a dose-proportional manner. The single-dose and steady-state pharmacokinetic parameters of empagliflozin were similar suggesting linear pharmacokinetics with respect to time. There were no clinically relevant differences in empagliflozin pharmacokinetics between healthy volunteers and patients with type 2 diabetes.
The pharmacokinetics of 5 mg empagliflozin twice daily and 10 mg empagliflozin once daily were compared in healthy subjects. Overall exposure (AUCss) of empagliflozin over a 24-hour period with empagliflozin 5 mg administered twice daily was similar to empagliflozin 10 mg administered once daily. As expected, empagliflozin 5 mg administered twice daily compared with 10 mg empagliflozin once daily resulted in lower Cmax and higher trough plasma empagliflozin concentrations (Cmin).
Administration of empagliflozin 25 mg after intake of a high-fat and high calorie meal resulted in slightly lower exposure; AUC decreased by approximately 16% and Cmax by approximately 37% compared to fasted condition. The observed effect of food on empagliflozin pharmacokinetics was not considered clinically relevant and empagliflozin may be administered with or without food. Similar results were obtained when Synjardy (empagliflozin/metformin) combination tablets were administered with high-fat and high calorie meal.
Distribution
The apparent steady-state volume of distribution was estimated to be 73.8 l based on the population pharmacokinetic analysis. Following administration of an oral [14C]-empagliflozin solution to healthy volunteers, the red blood cell partitioning was approximately 37% and plasma protein binding was 86%.
Biotransformation
No major metabolites of empagliflozin were detected in human plasma, as defined by at least 10% of total drug-related material, and the most abundant metabolites were three glucuronide conjugates (2-, 3-, and 6-O-glucuronide). In vitro studies suggested that the primary route of metabolism of empagliflozin in humans is glucuronidation by the uridine 5'-diphospho-glucuronosyltransferases UGT2B7, UGT1A3, UGT1A8, and UGT1A9.
Elimination
Based on the population pharmacokinetic analysis, the apparent terminal elimination half-life of empagliflozin was estimated to be 12.4 hours and apparent oral clearance was 10.6 l/hour. The inter-subject and residual variabilities for empagliflozin oral clearance were 39.1% and 35.8%, respectively. With once-daily dosing, steady-state plasma concentrations of empagliflozin were reached by the fifth dose. Consistent with the half-life, up to 22% accumulation, with respect to plasma AUC, was observed at steady-state. Following administration of an oral [14C]-empagliflozin solution to healthy volunteers, approximately 96% of the drug-related radioactivity was eliminated in faeces (41%) or urine (54%). The majority of drug-related radioactivity recovered in faeces was unchanged parent drug and approximately half of drug-related radioactivity excreted in urine was unchanged parent drug.
Special populations
Renal impairment
In patients with mild, moderate or severe renal impairment (creatinine clearance <30 - <90 ml/min) and patients with kidney failure/end stage renal disease (ESRD), AUC of empagliflozin increased by approximately 18%, 20%, 66%, and 48%, respectively compared to subjects with normal renal function. Peak plasma levels of empagliflozin were similar in subjects with moderate renal impairment and kidney failure/ESRD compared to patients with normal renal function. Peak plasma levels of empagliflozin were roughly 20% higher in subjects with mild and severe renal impairment as compared to subjects with normal renal function. The population pharmacokinetic analysis showed that the apparent oral clearance of empagliflozin decreased with a decrease in creatinine clearance leading to an increase in drug exposure.
Hepatic impairment
In subjects with mild, moderate, and severe hepatic impairment according to the Child-Pugh classification, AUC of empagliflozin increased approximately by 23%, 47%, and 75% and Cmax by approximately 4%, 23%, and 48%, respectively, compared to subjects with normal hepatic function.
Body Mass Index
Body mass index had no clinically relevant effect on the pharmacokinetics of empagliflozin based on the population pharmacokinetic analysis. In this analysis, AUC was estimated to be 5.82%, 10.4%, and 17.3% lower in subjects with BMI of 30, 35, and 45 kg/m², respectively, compared to subjects with a body mass index of 25 kg/m².
Gender
Gender had no clinically relevant effect on the pharmacokinetics of empagliflozin based on the population pharmacokinetic analysis.
Race
In the population pharmacokinetic analysis, AUC was estimated to be 13.5% higher in Asians with a body mass index of 25 kg/m² compared to non-Asians with a body mass index of 25 kg/m².
Elderly
Age did not have a clinically meaningful impact on the pharmacokinetics of empagliflozin based on the population pharmacokinetic analysis.
Paediatric population
A paediatric Phase 1 study examined the pharmacokinetics and pharmacodynamics of empagliflozin (5 mg, 10 mg and 25 mg) in children and adolescents ≥10 to <18 years of age with type 2 diabetes mellitus. The observed pharmacokinetic and pharmacodynamic responses were consistent with those found in adult subjects.
Metformin
Absorption
After an oral dose of metformin, tmax is reached in 2.5 hours. Absolute bioavailability of a 500 mg or 850 mg metformin hydrochloride tablet is approximately 50-60% in healthy subjects. After an oral dose, the non-absorbed fraction recovered in faeces was 20-30%. After oral administration, metformin absorption is saturable and incomplete. It is assumed that the pharmacokinetics of metformin absorption are non-linear. At the recommended metformin doses and dosing schedules, steady-state plasma concentrations are reached within 24 to 48 hours and are generally less than 1 microgram/ml. In controlled clinical trials, maximum metformin plasma levels (Cmax) did not exceed 5 microgram/ml, even at maximum doses.
Food decreases the extent and slightly delays the absorption of metformin. Following administration of a dose of 850 mg metformin hydrochloride, a 40% lower plasma peak concentration, a 25% decrease in AUC and a 35 minute prolongation of the time to peak plasma concentration were observed. The clinical relevance of these decreases is unknown.
Distribution
Plasma protein binding is negligible. Metformin partitions into erythrocytes. The blood peak is lower than the plasma peak and appears at approximately the same time. The red blood cells most likely represent a secondary compartment of distribution. The mean volume of distribution (Vd) ranged between 63-276 l. Biotransformation Metformin is excreted unchanged in the urine. No metabolites have been identified in humans.
Elimination
Renal clearance of metformin is >400 ml/min, indicating that metformin is eliminated by glomerular filtration and tubular secretion. Following an oral dose, the apparent terminal elimination half-life is approximately 6.5 hours.
When renal function is impaired, renal clearance is decreased in proportion to that of creatinine and thus the elimination half-life is prolonged, leading to increased levels of metformin in plasma.
Special populations
Paediatric population
Single dose study: after single doses of metformin hydrochloride 500 mg, paediatric patients have shown a similar pharmacokinetic profile to that observed in healthy adults.
Multiple-dose study: After repeated doses of 500 mg twice daily for 7 days in paediatric patients the peak plasma concentration (Cmax) and systemic exposure (AUC0-t) were approximately 33% and 40% lower, respectively, compared to diabetic adults who received repeated doses of 500 mg twice daily for 14 days. As the dose is individually titrated based on glycaemic control, this is of limited clinical relevance.
5.3. Preclinical safety data
Empagliflozin and metformin
General toxicity studies in rats of up to 13 weeks were performed with the combination of empagliflozin and metformin and did not reveal any additional target organs when compared to empagliflozin or metformin alone. Some responses were increased by the combination treatment, such as effects on renal physiology, electrolyte balance and acid/base state. However, only hypochloremia was considered adverse at exposures of approximately 9- and 3-times the clinical AUC exposure of the maximum recommended dose of empagliflozin and metformin, respectively.
An embryofetal development study in pregnant rats did not indicate a teratogenic effect attributed to the co-administration of empagliflozin and metformin at exposures of approximately 14-times the clinical AUC exposure of empagliflozin associated with the highest dose, and 4-times the clinical AUC exposure of metformin associated with the 2000 mg dose.
Empagliflozin
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, genotoxicity, fertility and early embryonic development.
In long term toxicity studies in rodents and dogs, signs of toxicity were observed at exposures greater than or equal to 10-times the clinical dose of empagliflozin. Most toxicity was consistent with secondary pharmacology related to urinary glucose loss and electrolyte imbalances including decreased body weight and body fat, increased food consumption, diarrhoea, dehydration, decreased serum glucose and increases in other serum parameters reflective of increased protein metabolism and gluconeogenesis, urinary changes such as polyuria and glucosuria, and microscopic changes including mineralisation in kidney and some soft and vascular tissues. Microscopic evidence of the effects of exaggerated pharmacology on the kidney observed in some species included tubular dilatation, and tubular and pelvic mineralisation at approximately 4-times the clinical AUC exposure of empagliflozin associated with the 25 mg dose.
Empagliflozin is not genotoxic.
In a 2-year carcinogenicity study, empagliflozin did not increase the incidence of tumours in female rats up to the highest dose of 700 mg/kg/day, which corresponds to approximately 72-times the maximal clinical AUC exposure to empagliflozin. In male rats, treatment-related benign vascular proliferative lesions (haemangiomas) of the mesenteric lymph node were observed at the highest dose, but not at 300 mg/kg/day, which corresponds to approximately 26-times the maximal clinical exposure to empagliflozin. Interstitial cell tumours in the testes were observed with a higher incidence in rats at 300 mg/kg/day and above, but not at 100 mg/kg/day which corresponds to approximately 18-times the maximal clinical exposure to empagliflozin. Both tumours are common in rats and are unlikely to be relevant to humans.
Empagliflozin did not increase the incidence of tumours in female mice at doses up to 1,000 mg/kg/day, which corresponds to approximately 62-times the maximal clinical exposure to empagliflozin. Empagliflozin induced renal tumours in male mice at 1,000 mg/kg/day, but not at 300 mg/kg/day, which corresponds to approximately 11-times the maximal clinical exposure to empagliflozin. The mode of action for these tumours is dependent on the natural predisposition of the male mouse to renal pathology and a metabolic pathway not reflective of humans. The male mouse renal tumours are considered not relevant to humans.
At exposures sufficiently in excess of exposure in humans after therapeutic doses, empagliflozin had no adverse effects on fertility or early embryonic development. Empagliflozin administered during the period of organogenesis was not teratogenic. Only at maternally toxic doses, empagliflozin also caused bent limb bones in the rat and increased embryofetal loss in the rabbit.
In pre- and postnatal toxicity studies in rats, reduced weight gain of offspring was observed at maternal exposures approximately 4-times the maximal clinical exposure to empagliflozin. No such effect was seen at systemic exposure equal to the maximal clinical exposure to empagliflozin. The relevance of this finding to humans is unclear.
In a juvenile toxicity study in the rat, when empagliflozin was administered from postnatal day 21 until postnatal day 90, non-adverse, minimal to mild renal tubular and pelvic dilation in juvenile rats was seen only at 100 mg/kg/day, which approximates 11-times the maximum clinical dose of 25 mg. These findings were absent after a 13 weeks drug-free recovery period.
Metformin
Preclinical data for metformin reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, or carcinogenic potential or reproductive toxicity. At dose levels of 500 mg/kg/day administered to Wistar Hannover rats, associated with 7-times the maximum recommended human dose (MRHD) of metformin, teratogenicity of metformin was observed, mostly evident as an increase in the number of skeletal malformations.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.