SYNTHROID Tablet Ref.[10711] Active ingredients: Levothyroxine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
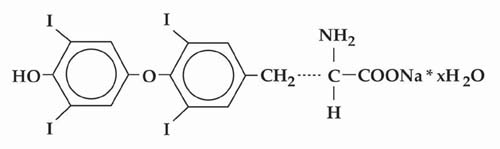
SYNTHROID (levothyroxine sodium tablets, USP) contain synthetic crystalline L-3,3',5,5'-tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic T4 is chemically identical to that produced in the human thyroid gland. Levothyroxine (T4) sodium has an empirical formula of C15H10I4N NaO4•H2O, molecular weight of 798.86 (anhydrous), and structural formula as shown:
SYNTHROID tablets for oral administration are supplied in the following strengths: 25 mcg, 50 mcg, 75 mcg, 88 mcg, 100 mcg, 112 mcg, 125 mcg, 137 mcg, 150 mcg, 175 mcg, 200 mcg, and 300 mcg. Each SYNTHROID tablet contains the inactive ingredients acacia, confectioner’s sugar (contains corn starch), lactose monohydrate, magnesium stearate, povidone, and talc. SYNTHROID tablets contain no ingredients made from a gluten-containing grain (wheat, barley, or rye). Each tablet strength meets USP Dissolution Test 3. Table 6 provides a listing of the color additives by tablet strength:
Table 6. SYNTHROID Tablets Color Additives:
| Strength (mcg) | Color additive(s) | |
|---|---|---|
| 25 | FD&C Yellow No. 6 Aluminum Lakea | |
| 50 | None | |
| 75 | FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake | |
| 88 | FD&C Blue No. 1 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lakea, D&C Yellow No. 10 Aluminum Lake | |
| 100 | D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lakea | |
| 112 | D&C Red No. 27 & 30 Aluminum Lake | |
| 125 | FD&C Yellow No. 6 Aluminum Lakea, FD&C Red No. 40 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake | |
| 137 | FD&C Blue No. 1 Aluminum Lake | |
| 150 | FD&C Blue No. 2 Aluminum Lake | |
| 175 | FD&C Blue No. 1 Aluminum Lake, D&C Red No. 27 & 30 Aluminum Lake | |
| 200 | FD&C Red No. 40 Aluminum Lake | |
| 300 | D&C Yellow No. 10 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lakea, FD&C Blue No. 1 Aluminum Lake | |
a Note – FD&C Yellow No. 6 is orange in color
| Dosage Forms and Strengths | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
SYNTHROID tablets are available as follows:
|
| How Supplied | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
SYNTHROID (levothyroxine sodium, USP) tablets are supplied as follows:
AbbVie Inc., North Chicago, IL 60064, U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| SYNTHROID | Brazil, Canada, Ecuador, Israel, Mexico, New Zealand, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.