TAZORAC Cream Ref.[27423] Active ingredients: Tazarotene
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
TAZORAC (tazarotene) Cream, 0.05% and 0.1% is for topical use and contains the active ingredient, tazarotene. Each gram of TAZORAC Cream, 0.05% and 0.1% contains 0.5 and 1 mg of tazarotene, respectively in a white cream base.
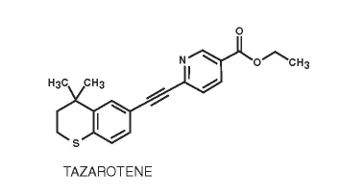
Tazarotene is a member of the acetylenic class of retinoids. Chemically, tazarotene is ethyl 6-[(4,4-dimethylthiochroman-6-yl) ethynyl]nicotinate. The compound has an empirical formula of C21H21NO2S and molecular weight of 351.46.
The structural formula is shown below:
TAZORAC Cream contains the following inactive ingredients: benzyl alcohol 1%; carbomer 1342; carbomer homopolymer type B; edetate disodium; medium chain triglycerides; mineral oil; purified water; sodium hydroxide; sodium thiosulfate; and sorbitan monooleate.
| Dosage Forms and Strengths |
|---|
|
Cream, 0.05% and 0.1%. Each gram of TAZORAC Cream, 0.05% and 0.1% contains 0.5 mg and 1 mg of tazarotene, respectively in a white cream base. |
| How Supplied | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
TAZORAC Cream is a white cream available in concentrations of 0.05% and 0.1%. It is supplied in a collapsible aluminum tube with a tamper-evident aluminum membrane over the opening and a white polypropylene screw cap, in 30 g and 60 g sizes.
Distributed by Almirall, LLC, Exton, PA 19341 |
Drugs
| Drug | Countries | |
|---|---|---|
| TAZORAC | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.