TAZVERIK Film coated tablet Ref.[10219] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
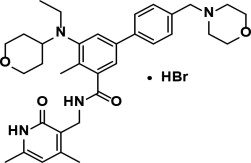
Tazemetostat is a methyltransferase inhibitor. Tazemetostat hydrobromide has the following chemical name: [1,1'-Biphenyl]-3-carboxamide, N-5[ethyl(tetrahydro-2H-pyran-4-yl)amino]-4-methyl-4'-(4-morpholinylmethyl)-, hydrobromide (1:1). The molecular formula of tazemetostat hydrobromide is C34H44N4O4∙HBr.
Tazemetostat hydrobromide has a molecular weight of 653.66 g/mol and the following structural formula:
Tazemetostat hydrobromide is a white to off-white solid that is slightly soluble in water and has pKa values of 5.26, 6.88, and 12.62. A saturated aqueous solution of tazemetostat hydrobromide has a pH of approximately 5 at ambient conditions.
TAZVERIK (tazemetostat) tablets for oral use contain 200 mg tazemetostat, equivalent to 228 mg tazemetostat hydrobromide.
Each tablet is film-coated and contains the following inactive ingredients in the tablet core: hydroxypropyl cellulose, lactose monohydrate, low-substituted hydroxypropyl cellulose, magnesium stearate, and sodium starch glycolate. The film-coat contains hypromellose, polyethylene glycol, red iron oxide, talc, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
Tablets: 200 mg film-coated, red, round, biconvex shape and debossed with “EZM 200” on one side and plain on the other. |
| How Supplied |
|---|
|
TAZVERIK 200 mg film-coated tablets are red, round, biconvex shape and debossed with “EZM 200” on one side and plain on the other. TAZVERIK is available in: Bottles of 240 tablets with a desiccant; NDC 72607-100-00 |
Drugs
| Drug | Countries | |
|---|---|---|
| TAZVERIK | Estonia, Japan, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.