TECARTUS Dispersion for infusion Ref.[49724] Active ingredients: Brexucabtagene autoleucel
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Kite Pharma EU B.V., Tufsteen 1, 2132 NT Hoofddorp, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other antineoplastic agents
ATC code: L01XL06
Mechanism of action
Tecartus, a CD19-directed genetically modified autologous T-cell immunotherapy, binds to CD19 expressing cancer cells and normal B cells. Following anti-CD19 CAR T-cell engagement with CD19 expressing target cells, the CD28 co-stimulatory domain and CD3-zeta signalling domain activate downstream signalling cascades that lead to T-cell activation, proliferation, acquisition of effector functions and secretion of inflammatory cytokines and chemokines. This sequence of events leads to killing of CD19-expressing cells.
Pharmacodynamic effects
In both ZUMA-2 and ZUMA-3, after Tecartus infusion, pharmacodynamic responses were evaluated over a 4-week interval by measuring transient elevation of cytokines, chemokines, and other molecules in blood. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, interferon-gamma (IFN-γ) and IL-2 receptor alpha were analysed. Peak elevation was generally observed within the first 8 days after infusion and levels generally returned to baseline within 28 days.
Due to the on target, off-tumour effect of Tecartus a period of B-cell aplasia may occur following treatment.
Translational analyses performed to identify associations between cytokine levels and incidence of CRS or neurologic events showed that higher levels (peak and AUC at 1 month) of multiple serum analytes, including IL-6, IL-10 and TNF-α, were associated with Grade 3 or higher neurologic adverse reactions and Grade 3 or higher CRS.
Clinical efficacy and safety
Relapsed or refractory MCL: ZUMA-2
The efficacy and safety of Tecartus in adult patients with relapsed or refractory MCL who had previously received anthracycline or bendamustine-containing chemotherapy, an anti CD20 antibody, and a Bruton’s tyrosine kinase inhibitor (BTKi) (ibrutinib or acalabrutinib), was evaluated in a phase 2 single-arm, open-label, multi-centre trial. Eligible patients also had disease progression after last regimen or refractory disease to the most recent therapy. Patients with active or serious infections, prior allogeneic haematopoietic stem cell transplantation (HSCT), detectable cerebrospinal fluid malignant cells or brain metastases, and any history of CNS lymphoma or CNS disorders were ineligible. In ZUMA-2, a total of, 74 patients were enrolled (i.e. leukapheresed) and 68 of these patients were treated with Tecartus. Three patients did not receive Tecartus due to manufacturing failure. Two other patients were not treated due to progressive disease (death) following leukapheresis. One patient was not treated with Tecartus after receiving lymphodepleting chemotherapy due to ongoing active atrial fibrillation. The full analysis set (FAS) was defined as all patients who underwent leukapheresis. A summary of the patient baseline characteristics is provided in Table 4.
Table 4. Summary of baseline characteristics for ZUMA-2:
| Category | All leukapheresed (FAS) (N=74) |
|---|---|
| Age (years) | |
| Median (min, max) | 65 (38, 79) |
| ≥65 | 58% |
| Male gender | 84% |
| Median number of prior therapies (min, max) | 3 (1; 5) |
| Relapsed/refractory subgroup | |
| Relapsed after auto-SCT | 42% |
| Refractory to last MCL therapy | 39% |
| Relapsed after last MCL therapy | 19% |
| Patients with disease stage IV | 86% |
| Patients with bone marrow involvement | 51% |
| Morphological characteristic | |
| Classical MCL | 54% |
| Blastoid MCL | 26% |
| Other | 1% |
| Unknown | 19% |
| Received bridging therapy | |
| Yes | 38% |
| No | 62% |
| Ki-67 IHC by central laboratory | |
| N | 49 |
| Median | 65% |
Auto-SCT, autologous stem cell transplant; IHC, immunohistochemistry; Max, maximum; MCL, mantle cell lymphoma; Min, minimum.
Tecartus was administered to patients as a single intravenous infusion at a target dose of 2 × 106 anti-CD19 CAR T cells/kg (maximum permitted dose: 2 × 108 cells) after lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m² intravenously and fludarabine 30 mg/m² intravenously, both given on the 5th, 4th, and 3rd day before treatment. Bridging therapy between leukapheresis and lymphodepleting chemotherapy was permitted to control disease burden.
For patients treated with Tecartus, the median time from leukapheresis to product release was 13 days (range: 9 to 20 days) and the median time from leukapheresis to Tecartus infusion was 27 days (range: 19 to 74 days, with the exception of one outlier of 134 days). The median dose was 2.0 × 106 anti-CD19 CAR T cells/kg. All patients received Tecartus infusion on day 0 and were hospitalized until day 7 at the minimum.
The primary endpoint was objective response rate (ORR) as determined by Lugano 2014 criteria by an independent review committee. Secondary endpoints included duration of response (DOR), overall survival (OS), progression free survival (PFS) and severity of adverse events.
For the primary analysis, the analysis set was defined a priori which consisted of the first 60 patients treated with Tecartus who were evaluated for response 6 months after the Week 4 disease assessment after Tecartus infusion. In this analysis set of 60 patients the ORR was 93% with a CR rate of 67%. The ORR was significantly higher than the prespecified historical control rate of 25% at a 1-sided significance level of 0.025 (p<0.0001).
The updated 24-month follow-up analyses of efficacy were conducted using the modified intent to treat (mITT) analysis set, which consisted of 68 patients treated with Tecartus. In the 24-month follow up analysis, the ORR and CR rates in the 68 patients in the mITT analysis set were 91% and 68% respectively.
Results in the FAS from both the primary analysis and 24-month follow-up analysis are shown in Table 5.
Table 5. Summary of efficacy results for ZUMA-2:
| Category | All leukaphereseda (FAS) (N=74) | |
|---|---|---|
| Primary Analysis | 24-month Follow-Up | |
| Objective response rate (ORR), n (%) [95% CI] | 62 (84%) [73.4, 91.3] | 62 (84%) [73.4, 91.3] |
| CR n (%) [95% CI] | 44 (59%) [47.4, 70.7] | 46 (62%) [50.1, 73.2] |
| PR n (%) [95% CI] | 18 (24%) [15.1, 35.7] | 16 (22%)[12.9, 32.7] |
| Duration of response (DOR)b | ||
| Median in months [95% CI] | NR [10.4, NE] | 28.2 (13.5, 47.1) |
| Rangec in months | 0.0+, 35.0+ | 0.0+, 53.0+ |
| Ongoing responses, CR+PR, CR, n (%)d | 32 (43%), 30 (41%) | 25 (34%), 25 (34%) |
| Progression free survival | ||
| Median, months [95% CI] | 16.2 [9.9, NE] | 24.0 (10.1, 48.2) |
| Overall survival | ||
| Median, months [95% CI] | NR [24.6, NE] | 47.4 (24.6, NE) |
| 6 month OS (%) [95% CI] | 83.6 [72.9, 90.3] | 83.6 [72.9, 90.3] |
| 12 month OS (%) [95% CI] | 76.6 [65.1, 84.8] | 76.7 [65.3, 84.8] |
| 24 month OS (%) [95% CI] | 66.5 [52.8, 77.1] | 63.0 [50.9, 70.3] |
| 30 month OS (%) [95% CI] | Not applicable | 56.2 (44.1, 66.7) |
| 36 month OS (%) [95% CI] | Not applicable | 53.9 (41.5, 64.8) |
| 54 month OS (%) [95% CI] | Not applicable | 38.7 (24.8, 52.4) |
| Median Follow-up in months (min, max) | 16.8 [7.2, 37.6] | 36.6 (27.3, 57.0) |
CI, confidence interval; CR, complete remission; FAS, full analysis set;; NE, not estimable; NR, not reached; OS, overall survival; PR, partial remission.
a Of the 74 patients that were enrolled (i.e. leukapheresed), 69 patients received lymphodepleting chemotherapy, and 68 patients received Tecartus.
b Among all responders. DOR is measured from the date of first objective response to the date of progression or death.
c A + sign indicates a censored value.
d At the data cutoff date. Percentages are calculated using the total number of patients in the analysis set as the denominator.
Figure 1. Kaplan Meier DOR in the FAS:
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Tecartus in all subsets of the paediatric population in treatment of MCL (see section 4.2 for information on paediatric use).
Relapsed or refractory B-cell precursor ALL: ZUMA-3
A Phase 2, open-label, multicenter trial evaluated the efficacy and safety of Tecartus in adult patients with relapsed or refractory B-precursor ALL. Relapsed or refractory was defined as one of the following: primary refractory; first relapse following a remission lasting ≤12 months; relapsed or refractory after second-line or higher therapy; relapsed or refractory after allogeneic stem cell transplant (allo-SCT) (provided the transplant occurred ≥100 days prior to enrollment and that no immunosuppressive medications were taken ≤4 weeks prior to enrollment). The study excluded patients with active or serious infections, active graft-vs-host disease, and any history of CNS disorders. Patients with CNS-2 disease without clinically evident neurologic changes were eligible. In ZUMA-3 Phase 2, a total of 71 patients were enrolled (i.e. leukapheresed) and 55 patients were treated with Tecartus. Six patients did not receive Tecartus due to manufacturing failure. Eight other patients were not treated, primarily due to AEs following leukapheresis. Two patients who underwent leukapheresis and received lymphodepleting chemotherapy were not treated with Tecartus; one patient experienced bacteremia and neutropenic fever and the other patient did not meet eligibility criteria after lymphodepleting chemotherapy. The FAS included all patients who underwent leukapheresis and the modified intent to treat (mITT) analysis set includes all patients leukapheresed and treated with Tecartus in Phase 2. A summary of patient baseline characteristics is provided in Table 6.
Table 6. Summary of baseline characteristics for ZUMA-3 Phase 2:
| Category | All leukapheresed (FAS) (N=71) | All treated (mITT) (N=55) |
|---|---|---|
| Age (years) | ||
| Median (min, max) | 44 (19 to 84) | 40 (19 to 84) |
| Male gender | 58% | 60% |
| White ethnicity | 72% | 67% |
| Primary refractory disease | 30% | 33% |
| Relapsed/refractory disease after ≥2 lines of therapy | 76% | 78% |
| Relapse with first remission ≤12 months | 28% | 29% |
| Number of Lines of Prior Therapy | ||
| Median (min, max) | 2 (1 to 8) | 2 (1 to 8) |
| ≥3 | 48% | 47% |
| Prior Therapies | ||
| Allo-SCT | 39% | 42% |
| Blinatumomab | 46% | 45% |
| Inotuzumab | 23% | 22% |
| Philadelphia chromosome (Ph+) | 27% | 27% |
Allo-SCT, allogenic stem cell transplant; Max, maximum; Min, minimum
Following lymphodepleting chemotherapy, Tecartus was administered to patients as a single intravenous infusion at a target dose of 1 × 106 anti-CD19 CAR T cells/kg (maximum permitted dose: 1 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 900 mg/m² intravenously over 60 mins on the 2nd day before Tecartus infusion and fludarabine 25 mg/m² intravenously over 30 mins on the 4th, 3rd, and 2nd day before Tecartus infusion. Of the 55 patients who recived Tecartus, 51 patients received bridging therapy between leukapheresis and lymphodepleting chemotherapy to control disease burden.
The median time from leukapheresis to product delivery was 16 days (range: 11 to 42 days) and the median time from leukapheresis to Tecartus infusion was 29 days (range: 20 to 60 days). The median dose was 1.0 × 106 anti-CD19 CAR T cells/kg. All patients received Tecartus infusion on day 0 and were hospitalized until day 7 at the minimum.
The primary endpoint was overall complete remission rate (OCR) (complete remission [CR] + complete remission with incomplete hematologic recovery [CRi]) in patients treated with Tecartus as determined by an independent review. In the 55 patients treated with Tecatrus (mITT), the OCR rate was 70.9% with a CR rate of 56.4% (Table 7), which was significantly greater than the prespecified control rate of 40%. Among the 39 patients who achieved a CR or CRi, the median time to response was 1.1 months (range: 0.85 to 2.99 months).
All treated patients had potential follow-up for ≥18 months with a median follow-up time of 20.5 months (95% CI: 0.3, 32.6 months) and a median follow-up time for OS of 24.0 months (95% CI: 23.3, 24.6).
Table 7. Summary of efficacy results for ZUMA3 Phase 2:
| FAS N=71 | mITTa N=55 | |
|---|---|---|
| OCR rate (CR + CRi) n (%) [95% CI] | 39 (54.9) [43, 67] | 39 (70.9) [57.0, |
| CR rate, n (%) [95% CI] | 31 (43.7) [32, 56] | 31 (56.4) [42.0, 70.0] |
| Minimal Residual Disease (MRD) negative rate among OCR (CR or CRi) patients, n (%) | n=39 38 (97%) | n=39 38 (97%) |
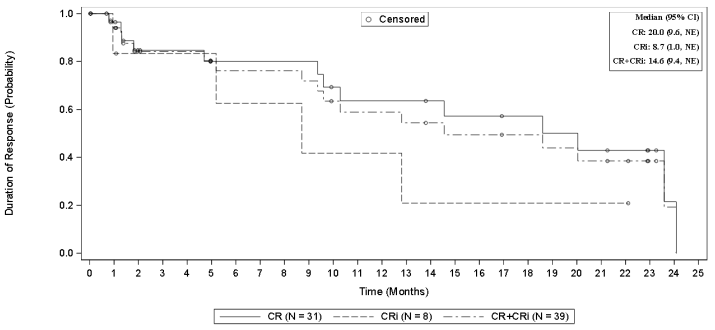
| Duration of Remission, median in months [95% CI]b Median range in months | 14.6 [9.4, NE]c (0.03+, 24.08+) | 14.6 [9.4, NE]c (0.03+, 24.08+) |
CI, confidence interval; CR, complete remission; NE, not estimable
a Of the 71 patients that were enrolled (and leukapheresed), 57 patients received conditioning chemotherapy, and 55 patients received Tecartus.
b Subjects were censored at their last evaluable disease assessment before initiation of a new anticancer therapy (excluding resumption of a tyrosine kinase inhibitor) or allo-SCT to exclude any contribution that the new therapy might have on DOR that could confound the contribution of KTE-X19. The results of the analyses that did not censor for subsequent allo-SCT or the initiation of new anti-cancer therapy were consistent with the analyses that did censor the events.
c The duration of remission was defined only for subjects achieving an OCR, therefore the results of the analysis in the FAS and mITT were identical.
Figure 2. Kaplan Meier DOR in the mITT Analysis Seta:
a The DOR was defined only for subjects achieving an OCR, therefore the results of the analysis in the FAS and mITT were identical.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Tecartus in one or more subsets of the B-cell ALL paediatric population and waived the obligation to submit the results of studies with Tecartus for the treatment of ALL in the paediatric population weighing less than 6kg. See section 4.2 for information on paediatric use.
Conditional Approval
This medicinal product has been authorised under a so-called ‘conditional approval’ scheme.
This means that further evidence on this medicinal product is awaited in both the MCL and ALL patient population.
The European Medicines Agency will review new information on this medicinal product at least every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
Cellular kinetics
Mantle cell lymphoma
Following infusion of 2 × 106 anti-CD19 CAR T cells/kg of Tecartus in ZUMA-2, anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 3 months. Peak levels of anti-CD19 CAR T cells occurred within the first 7 to 15 days after the infusion.
Among patients with MCL, the number of anti-CD19 CAR T cells in blood was associated with objective response (CR or PR) (Table 8).
Table 8. Summary of brexucabtagene autoleucel pharmacokinetics in ZUMA-2:
| Number of anti-CD19 CAR T cell | Responding patients (CR or PR) (N=63) | Non-responding patients (N=5) | P-Value |
|---|---|---|---|
| Peak (cells/μL) Median [min; max], n | 97.52 [0.24, 2 589.47], 62 | 0.39 [0.16, 22.02], 5 | 0.0020 |
| AUC0–28 (cells/μL·day) Median [min; max], n | 1 386.28 [3.83 to 2.77 × 104], 62 | 5.51 [1.81, 293.86], 5 | 0.0013 |
P-value is calculated by Wilcoxon test.
Median peak anti-CD19 CAR T-cell values were 74.08 cells/μL in MCL patients ≥65 years of age (n=39) and 112.45 cells/μL in MCL patients <65 years of age (n=28). Median anti-CD19 CAR T-cell AUC values were 876.48 cells/μL∙day in MCL patients ≥65 years of age and 1 640.21 cells/μL∙day in MCL patients <65 years of age.
Acute lymphoblastic leukaemia
Following infusion of a target dose of 1 × 106 anti-CD19 CAR T cells/kg of Tecartus in ZUMA-3 (Phase 2), anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 3 months. Median time to peak levels of anti-CD19 CAR T cells was within the first 15 days after Tecartus infusion.
A summary of the Tecartus pharmacokinetics over time, based on central assessment by overall response, is provided in Table 9.
Table 9. Summary of brexucabtagene autoleucel pharmacokinetics in ZUMA-3 Phase 2:
| Number of anti-CD19 CAR T cell | Patients with overall complete remission (CR/CRi) (N=39) | Patients with non- complete remissiona (N=16) | P-Value |
|---|---|---|---|
| Peak (cells/μL) Median [min; max], n | 38.35 [1.31, 1 533.4], 36b | 0.49 [0.00, 183.50], 14b | 0.0001c |
| AUC0–28 (cells/μL·day) Median [min; max], n | 424.03 [14.12 to 19 390.42], 36b | 4.12 [0.00, 642.25], 14b | 0.0001c |
a Three of 39 subjects who achieved CR or CRi and 2 of 16 subjects who were non-CR/CRi had no anti-CD19 CAR Tcell data at any postinfusion visit.
b Noncomplete remission includes all non-CR/CRi subjects whose response is classified incomplete remission response with partial hematologic recovery, blast-free hypoplastic or aplastic bone marrow (N=4), partial response (N=0), no response (N=9), or not evaluable (N=3).
c P-value is calculated by Wilcoxon test.
Median peak anti-CD19 CAR T-cell values were 34.8 cells/μL in ALL patients ≥65 years of age (n=8) and 17.4 cells/μL in ALL patients <65 years of age (n=47). Median anti-CD19 CAR T-cell AUC values were 425.0 cells/μL∙day in ALL patients ≥65 years of age and 137.7 cells/μL∙day in ALL patients <65 years of age.
In MCL and ALL patients, gender had no significant impact on AUCDay 0–28 and Cmax of Tecartus.
Studies of Tecartus in patients with hepatic and renal impairment were not conducted.
5.3. Preclinical safety data
Tecartus comprises engineered human T cells; therefore, there are no representative in vitro assays, ex vivo models, or in vivo models that can accurately address the toxicological characteristics of the human product. Hence, traditional toxicology studies used for medicinal product development were not performed.
No carcinogenicity or genotoxicity studies have been conducted.
No studies have been conducted to evaluate the effects of this treatment on fertility, reproduction, and development.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.