TELZIR Film-coated tablet Ref.[8776] Active ingredients: Fosamprenavir
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: ViiV Healthcare BV, Huis ter Heideweg 62, 3705 LZ Zeist, Netherlands
Contraindications
Hypersensitivity to fosamprenavir, amprenavir, or ritonavir, or to any of the excipients listed in section 6.1.
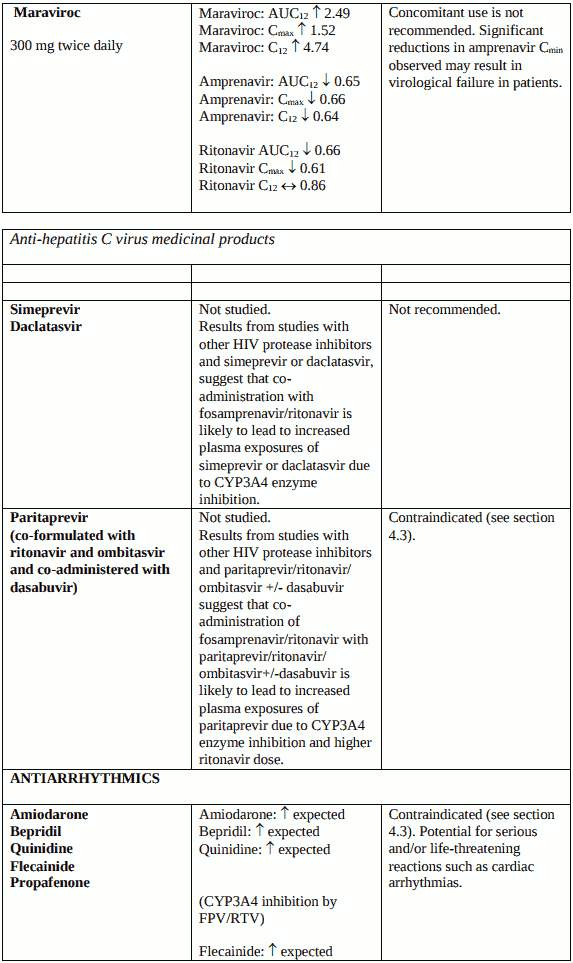
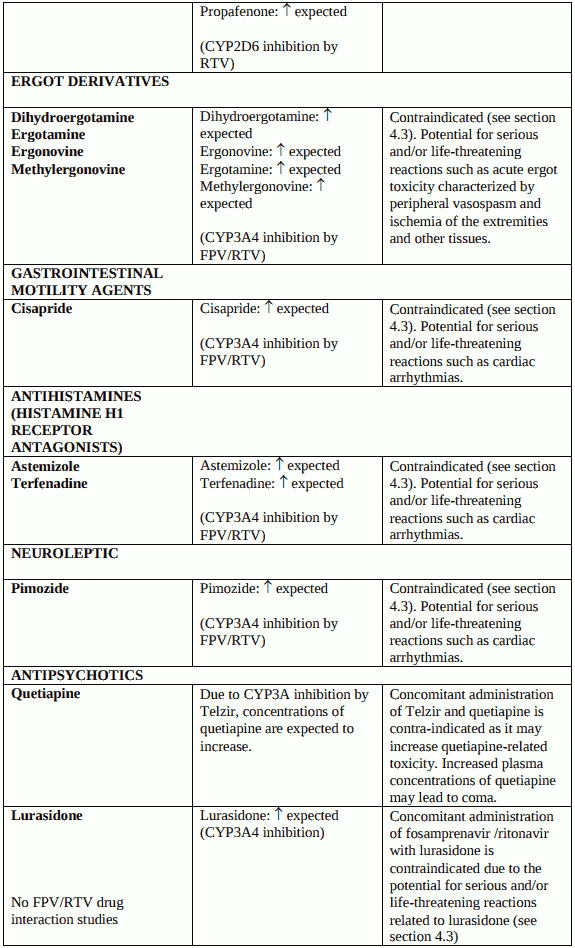
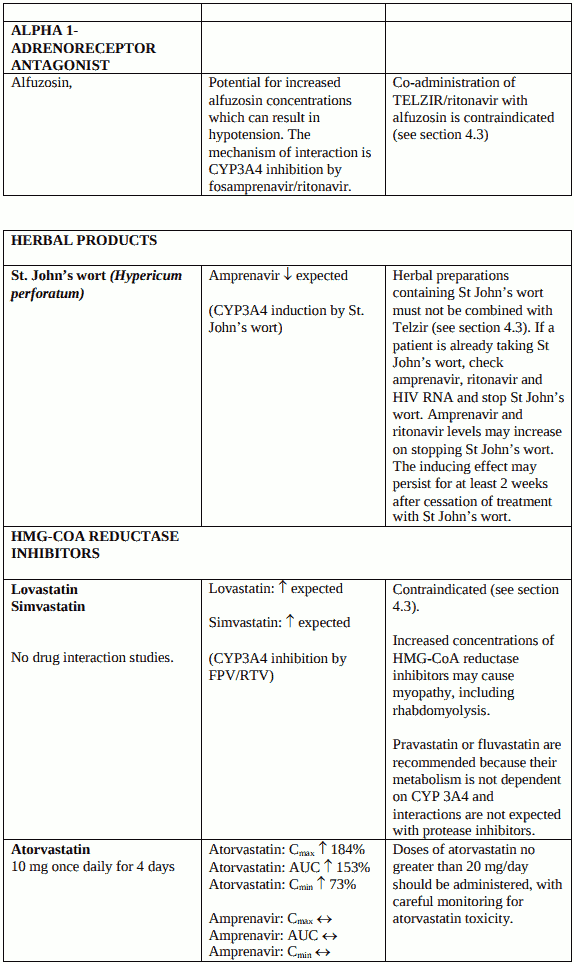
Telzir must not be administered concurrently with medicinal products with narrow therapeutic windows that are substrates of cytochrome P450 3A4 (CYP3A4), e.g. alfuzosin, amiodarone, astemizole, bepridil, cisapride, dihydroergotamine, ergotamine, pimozide, quetiapine, quinidine, terfenadine, oral midazolam (for caution on parenterally administered midazolam, see section 4.5), oral triazolam, sildenafil used for the treatment of pulmonary arterial hypertension (for use of sildenafil in patients with erectile dysfunction, see sections 4.4 and 4.5).
Co-administration of the antipsychotic medicinal product lurasidone and fosamprenavir/ritonavir (FPV/RTV) is contraindicated (see section 4.5).
Co-administration of paritaprevir and fosamprenavir/ritonavir (FPV/RTV) is contraindicated due to the expected increase of paritaprevir exposure and the lack of clinical data assessing the magnitude of this increase (see section 4.5).
Concomitant use of Telzir with simvastatin or lovastatin is contraindicated because of increased plasma concentrations of lovastatin and simvastatin which can increase the risk of myopathy, including rhabdomyolysis (see section 4.5). Telzir with ritonavir must not be co-administered with medicinal products with narrow therapeutic windows that are highly dependent on CYP2D6 metabolism, e.g. flecainide and propafenone (see section 4.5).
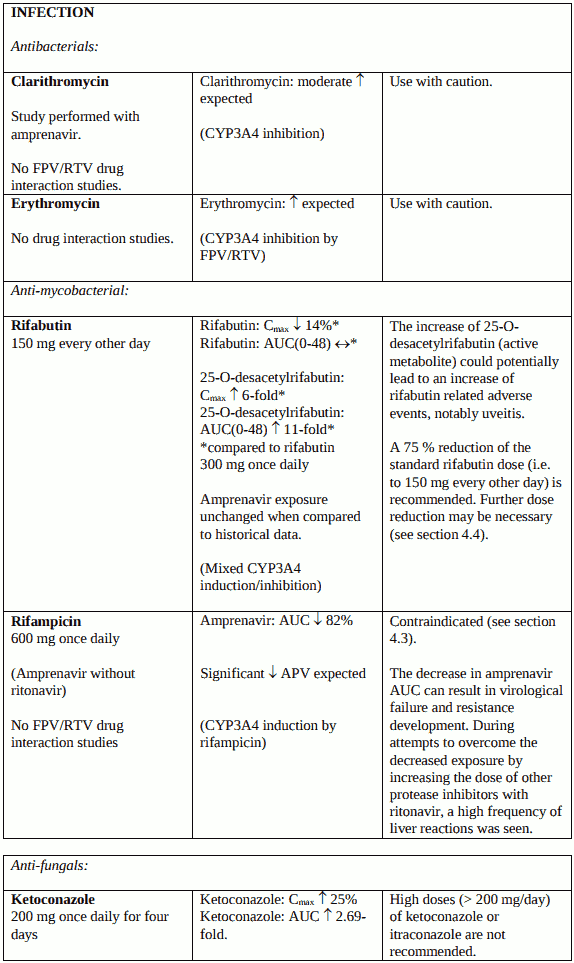
Combination of rifampicin with Telzir with concomitant low-dose ritonavir is contraindicated (see section 4.5).
Herbal preparations containing St John’s wort (Hypericum perforatum) must not be used while taking Telzir due to the risk of decreased plasma concentrations and reduced clinical effects of amprenavir (see section 4.5).
Special warnings and precautions for use
While effective viral suppression with antiretroviral therapy has been proven to substantially reduce the risk of sexual transmission, a residual risk cannot be excluded. Precautions to prevent transmission should be taken in accordance with national guidelines.
Patients should be advised that treatment with Telzir, or any other current antiretroviral therapy, does not cure HIV and that they may still develop opportunistic infections and other complications of HIV infection.
Fosamprenavir contains a sulphonamide moiety. The potential for cross-sensitivity between medicinal products in the sulphonamide class and fosamprenavir is unknown. In the pivotal studies of Telzir, in patients receiving fosamprenavir with ritonavir there was no evidence of an increased risk of rashes in patients with a history of sulphonamide allergy versus those who did not have a sulphonamide allergy. Yet, Telzir should be used with caution in patients with a known sulphonamide allergy.
Co-administration of Telzir 700 mg twice daily with ritonavir in doses greater than 100 mg twice daily has not been clinically evaluated. The use of higher ritonavir doses might alter the safety profile of the combination and therefore is not recommended.
Liver disease
Telzir with ritonavir should be used with caution and at reduced doses in adults with mild, moderate, or severe hepatic impairment (see section 4.2).
Patients with chronic hepatitis B or C and treated with combination antiretroviral therapy are at an increased risk of severe and potentially fatal hepatic adverse reactions. In case of concomitant antiviral therapy for hepatitis B or C, please refer also to the relevant Summary of Product Characteristics for these medicinal products.
Patients with pre-existing liver dysfunction, including chronic active hepatitis, have an increased frequency of liver function abnormalities during combination antiretroviral therapy and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
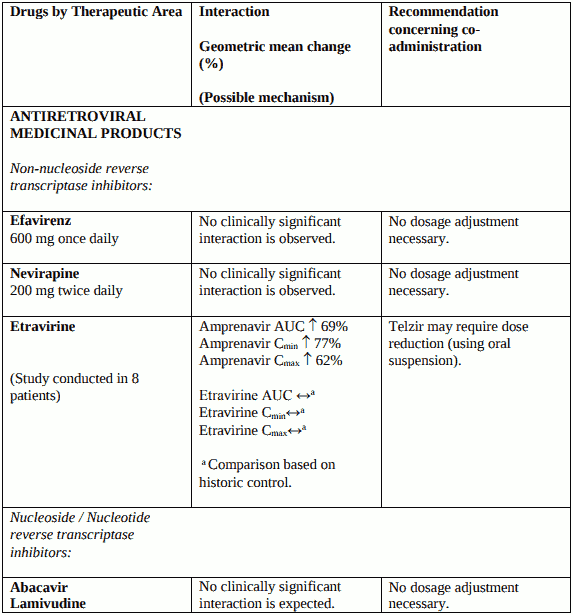
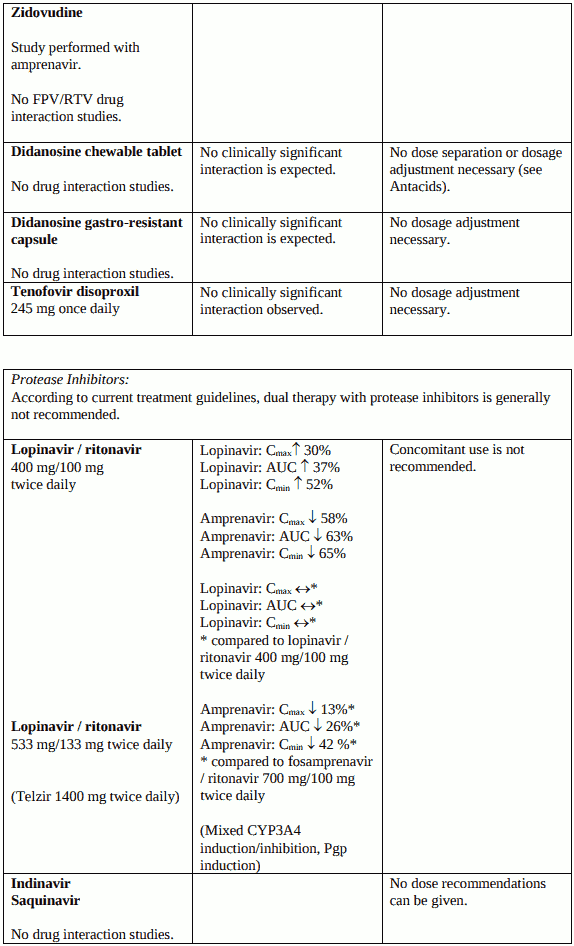
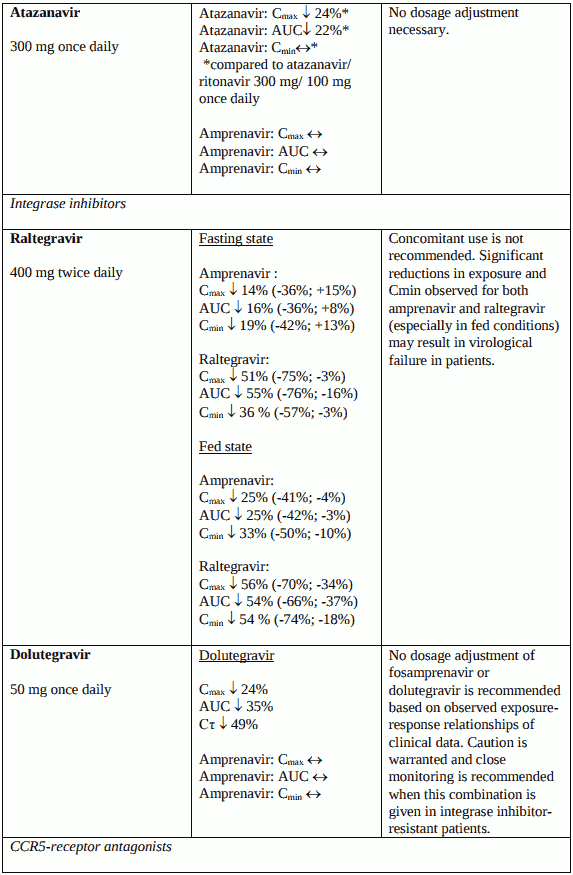
Medicinal products – interactions
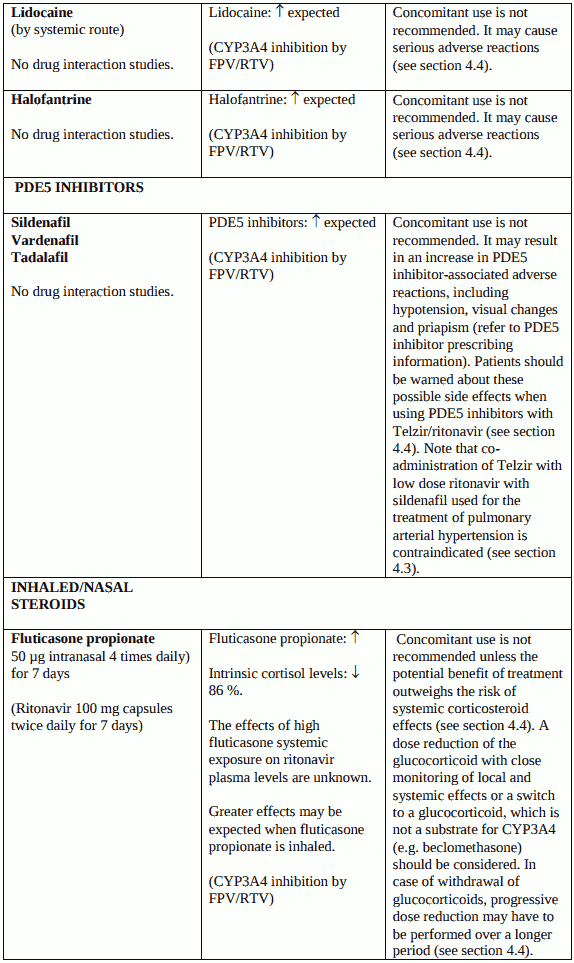
The use of Telzir concomitantly with halofantrine or lidocaine (systemic) is not recommended (see section 4.5).
PDE5 inhibitors used for the treatment of erectile dysfunction: The use of Telzir concomitantly with PDE5 inhibitors (e.g. sildenafil, tadalafil, vardenafil) is not recommended (see section 4.5). Co-administration of Telzir with low dose ritonavir and these medicinal products is expected to substantially increase their concentrations and may result in PDE5 inhibitor-associated adverse events such as hypotension, visual changes and priapism (see section 4.5). Note that co-administration of Telzir with low dose ritonavir with sildenafil used for the treatment of pulmonary arterial hypertension is contraindicated (see section 4.3).
A reduction in the rifabutin dosage by at least 75 % is recommended when administered with Telzir with ritonavir. Further dose reduction may be necessary (see section 4.5).
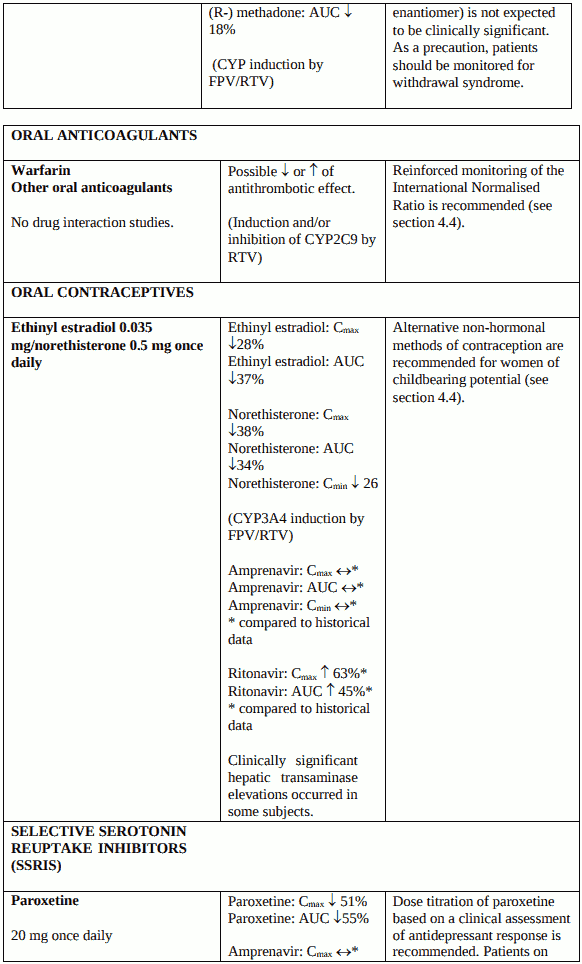
Because there may be an increased risk of hepatic transaminase elevations and hormonal levels may be altered with co-administration of fosamprenavir, ritonavir and oral contraceptives, alternative nonhormonal methods of contraception are recommended for women of childbearing potential (see section 4.5). No data are available on the co-administration of fosamprenavir and ritonavir with oestrogens and/or progestogens when used as hormonal replacement therapies. The efficacy and safety of these therapies with fosamprenavir and ritonavir has not been established.
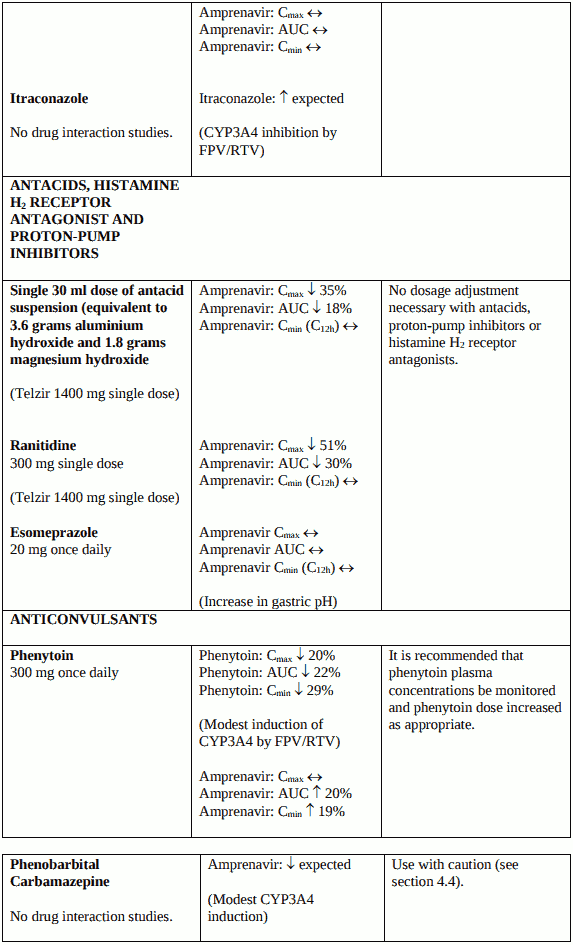
Anticonvulsants (carbamazepine, phenobarbital) should be used with caution. Telzir may be less effective due to decreased amprenavir plasma concentrations in patients taking these medicinal products concomitantly (see section 4.5).
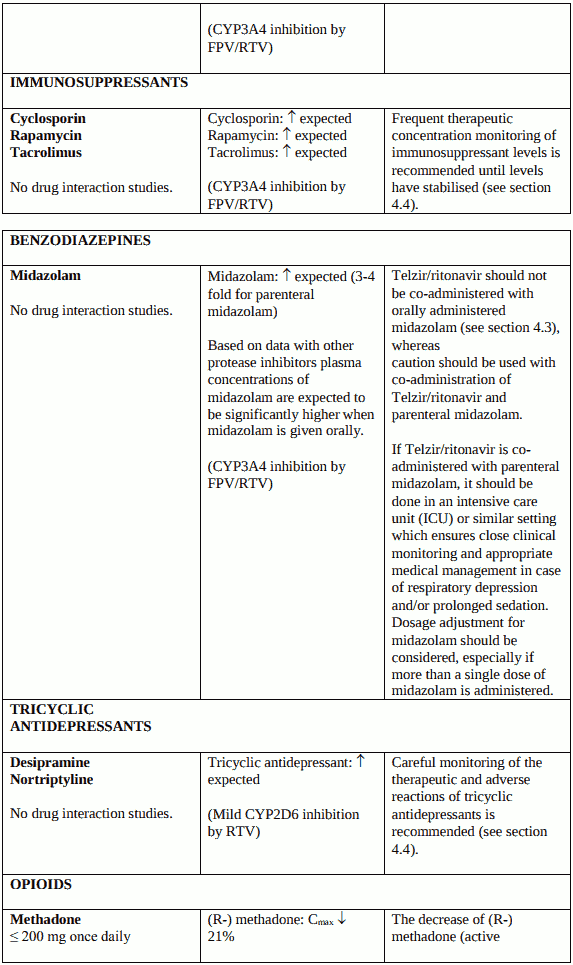
Therapeutic concentration monitoring is recommended for immunosuppressant medicinal products (cyclosporine, tacrolimus, rapamycin) when co-administered with Telzir (see section 4.5).
Therapeutic concentration monitoring is recommended for tricyclic antidepressants (e.g. desipramine and nortriptyline) when coadministered with Telzir (see section 4.5).
When warfarin or other oral anticoagulants are coadministered with Telzir a reinforced monitoring of INR (International Normalised Ratio) is recommended (see section 4.5).
Concomitant use of Telzir with ritonavir and fluticasone or other glucocorticoids that are metabolised by CYP3A4 is not recommended unless the potential benefit of treatment outweighs the risk of systemic corticosteroid effects, including Cushing’s syndrome and adrenal suppression (see section 4.5).
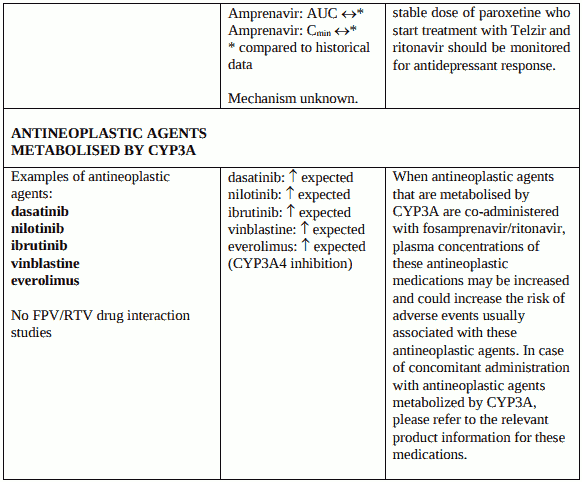
Co-administration of fosamprenavir/ritonavir with other antineoplastics metabolised by CYP3A (for example dasatinib, nilotinib, ibrutinib, vinblastine and everolimus) may increase concentrations of these medicinal products, potentially increasing the risk of adverse events usually associated with these agents. Please refer to the relevant product information for these medications (see section 4.5).
Hepatitis C virus (HCV) Direct-Acting Antivirals: When hepatitis C virus direct-acting antiviral (DAA) drugs, which are metabolised by CYP3A4 or are inducers/inhibitors of CYP3A4, are coadministered with fosamprenavir/ritonavir, altered plasma concentrations of medications are expected due to inhibition or induction of CYP3A4 enzyme activity (see sections 4.3 and 4.5).
Rash / cutaneous reactions
Most patients with mild or moderate rash can continue Telzir. Appropriate antihistamines (e.g. cetirizine dihydrochloride) may reduce pruritus and hasten the resolution of rash. Severe and life-threatening skin reactions, including Stevens-Johnson syndrome, were reported in less than 1% of patients included in the clinical development programme. Telzir should be permanently discontinued in case of severe rash, or in case of rash of moderate intensity with systemic or mucosal symptoms (see section 4.8).
Haemophiliac patients
There have been reports of increased bleeding including spontaneous skin haematomas and haemarthroses in haemophiliac patients type A and B treated with protease inhibitors (PIs). In some patients administration of factor VIII was necessary. In more than half of the reported cases, treatment with protease inhibitors was continued, or reintroduced if treatment had been discontinued. A causal relationship has been evoked, although the mechanism of action has not been elucidated. Haemophiliac patients should therefore be informed of the possibility of increased bleeding.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and life style. For lipids, there is in some cases evidence for a treatment effect, while for weight gain there is no strong evidence relating this to any particular treatment. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Immune Reactivation Syndrome
In HIV-infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterium infections, and Pneumocystis carinii pneumonia. Any inflammatory symptoms should be evaluated andtreatment instituted when necessary. Autoimmune disorders (such as Graves' disease) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and can occur many months after initiation of treatment.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported particularly in patients with advanced HIV-disease and/or long-term exposure to CART. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Interaction with other medicinal products and other forms of interaction
When fosamprenavir and ritonavir are co-administered, the ritonavir metabolic drug interaction profile may predominate because ritonavir is a more potent CYP3A4 inhibitor. The full prescribing information for ritonavir must therefore be consulted prior to initiation of therapy with Telzir with ritonavir. Ritonavir also inhibits CYP2D6 but to a lesser extent than CYP3A4. Ritonavir induces CYP3A4, CYP1A2, CYP2C9 and glucuronosyl transferase.
Additionally, both amprenavir, the active metabolite of fosamprenavir, and ritonavir are primarily metabolised in the liver by CYP3A4. Therefore, any medicinal products that either share this metabolic pathway or modify CYP3A4 activity may modify the pharmacokinetics of amprenavir and ritonavir. Similarly administration of fosamprenavir with ritonavir may modify the pharmacokinetics of other active substances that share this metabolic pathway.
Interaction studies have only been performed in adults.
Unless otherwise stated, studies detailed below have been performed with the recommended dosage of fosamprenavir/ritonavir (i.e. 700/100 mg twice daily), and the interaction was assessed under steadystate conditions where drugs were administered for 10 to 21 days.
Fertility, pregnancy and lactation
Pregnancy
As a general rule, when deciding to use antiretroviral agents for the treatment of HIV infection in pregnant women and consequently for reducing the risk of HIV vertical transmission to the newborn, the animal data (see section 5.3) as well as the clinical experience in pregnant women should be taken into account.
There is limited clinical experience (less than 300 pregnancy outcomes) from the use of fosamprenavir in pregnant women. Placental transfer of amprenavir has been shown to occur in humans.
In animal studies at systemic plasma exposures (AUC) to amprenavir lower than therapeutic exposure in patients treated with Telzir, some developmental toxicity was observed (see section 5.3). In view of the low exposure in reproductive toxicity studies, the potential developmental toxicity of Telzir has not been fully determined.
Telzir should be used during pregnancy only if the potential benefit justifies the potential risk to the foetus.
Breast-feeding
Amprenavir-related material was found in rat milk, but it is not known whether amprenavir is excreted in human milk. Rat pups exposed pre and post-natally to amprenavir and fosamprenavir showed developmental toxicity (see section 5.3).
It is recommended that HIV-infected women must not breast-feed under any circumstances to avoid transmission of HIV.
Fertility
No human data on the effect of fosamprenavir on fertility are available. In rats, there was no major effect on fertillty or reproductive performance with fosamprenavir (see section 5.3).
Effects on ability to drive and use machines
No studies on the effects of Telzir in combination with ritonavir on the ability to drive and use machines have been performed. The adverse reaction profile of Telzir should be borne in mind when considering the patient’s ability to drive or operate machinery (see section 4.8).
Undesirable effects
Summary of safety profile
The adverse reaction profile was similar across all the respective adult studies: antiretroviral naïve patients (APV30002, ESS100732), protease inhibitor experienced (twice daily dosing, APV30003) patients. This is based on safety data from a total of 864 patients exposed to fosamprenavir/ritonavir in these three studies.
The most frequently (>5% of adult subjects treated) reported adverse reactions with fosamprenavir/ritonavir combination were gastrointestinal reactions (nausea, diarrhoea, abdominal pain and vomiting) and headache. Most adverse reactions associated with fosamprenavir/ritonavir combination therapies were mild to moderate in severity, early in onset and rarely treatment limiting. More serious adverse reactions such as serious skin rashes and hepatic transaminase elevations have also been reported (cf paragraph Description of selected adverse reactions).
Tabulated summary of adverse reactions
Adverse reactions are listed by MedDRA system organ class and absolute frequency. Frequencies are defined as: Very common (≥1/10), Common (≥1/100 to <1/10), Uncommon (≥1/1,000 to <1/100), Rare (≥1/10,000 to <1/1,000) or Very rare (<1/10,000), or Not known.
Frequency categories for the reactions below have been based on clinical trials and postmarketing data.
Most of the adverse reactions below were reported from three large clinical studies in adults, where the adverse events were of at least moderate intensity (Grade 2 or more) occurring in at least 1% of patients and reported by investigators as being attributable to the medicinal products used in the studies.
Nervous system disorders
Common: Headache, dizziness, oral paraesthesia
Gastrointestinal disorders
Very common: Diarrhoea
Common: Loose stools, nausea, vomiting, abdominal pain
Skin and subcutaneous tissue disorders
Rare: Stevens Johnson syndrome
Uncommon: Angioedema
Common: Rash (see text below “rash/cutaneous reactions”)
General disorders and administration site conditions
Common: Fatigue
Investigations
Very common: Blood cholesterol increased
Common: Blood triglycerides increased, Alanine aminotransferase increased, Aspartate aminotransferase increased, Lipase increased
Description of selected adverse reactions
Rash/cutaneous reactions
Erythematous or maculopapular cutaneous eruptions, with or without pruritus, may occur during therapy. The rash generally will resolve spontaneously without the necessity of discontinuing treatment with the fosamprenavir with ritonavir.
Severe or life-threatening cases of rash, including Stevens-Johnson syndrome are rare. Fosamprenavir with ritonavir therapy should be definitively stopped in case of severe rash or in case of rash of mild or moderate intensity associated with systemic or mucosal signs (see section 4.4).
Clinical chemistry abnormalities
Clinical chemistry abnormalities (Grade 3 or 4) potentially related to treatment with fosamprenavir with ritonavir and reported in greater than or equal to 1% of adult patients, included: increased ALT (common), AST (common), serum lipase (common) and triglycerides (common).
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Rhabdomyolysis
An increase in CPK, myalgia, myositis, and rarely, rhabdomyolysis, have been reported with protease inhibitors, more specifically in association with nucleoside analogues.
Immune Reactivation Syndrome
In HIV-infected patients with severe immune deficiency at the time of initiation of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic infections may arise. Autoimmune disorders (such as Graves' disease) have also been reported to occur in the setting of immune reactivation; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment (see section 4.4).
Osteonecrosis
Cases of osteonecrosis have been reported, particularly in patients with generally acknowledged risk factors, advanced HIV disease or long-term exposure to CART. The frequency of this is unknown (see section 4.4).
Paediatric / other populations
Children and adolescents
The adverse reaction profile in children and adolescents is based on integrated safety data from two studies (APV29005 Week 24 data and APV20003 Week 168 data [final data]) in which 158 HIV-1 infected subjects 2 to 18 years of age received fosamprenavir with ritonavir with background nucleoside reverse transcriptase inhibitor therapy (see section 5.1 for information on dosing regimens applied for each age group). 79% of subjects received greater than 48 weeks of exposure.
Overall the safety profile in these 158 children and adolescents was similar to that observed in the adult population. Vomiting occurred more frequently amongst paediatric patients. Drug-related adverse reactions were more common in APV20003 (57%) where subjects received once daily fosamprenavir/ritonavir when compared to APV29005 (33%) where subjects received twice daily fosamprenavir/ritonavir.
No new safety concerns were identified from analyses of 48 week data from studies APV29005 or APV20002, in which 54 subjects 4 weeks to <2 years of age received twice daily fosamprenavir/ritonavir with background nucleoside reverse transcriptase inhibitor therapy and 5 subjects received only single doses of fosamprenavir with or without ritonavir.
Haemophiliac patients
There have been reports of increased spontaneous bleeding in haemophiliac patients receiving antiretroviral protease inhibitors (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.