TEMODAL Powder for solution for infusion Ref.[8453] Active ingredients: Temozolomide
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Merck Sharp & Dohme B.V., Waarderweg 39, 2031 BN Haarlem, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents – Other alkylating agents
ATC code: L01AX03
Mechanism of action
Temozolomide is a triazene, which undergoes rapid chemical conversion at physiologic pH to the active monomethyl triazenoimidazole carboxamide (MTIC). The cytotoxicity of MTIC is thought to be due primarily to alkylation at the O6 position of guanine with additional alkylation also occurring at the N7 position. Cytotoxic lesions that develop subsequently are thought to involve aberrant repair of the methyl adduct.
Clinical efficacy and safety
Newly-diagnosed glioblastoma multiforme
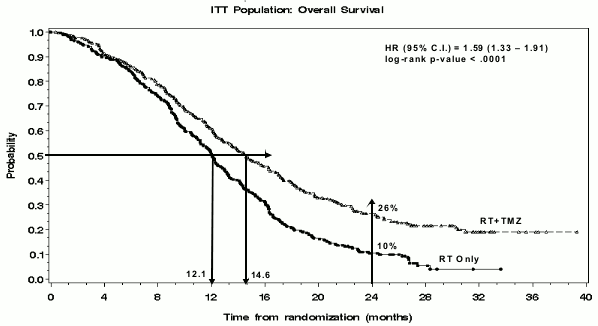
A total of 573 patients were randomised to receive either TMZ + RT (n=287) or RT alone (n=286). Patients in the TMZ + RT arm received concomitant TMZ (75 mg/m²) once daily, starting the first day of RT until the last day of RT, for 42 days (with a maximum of 49 days). This was followed by monotherapy TMZ (150-200 mg/m²) on Days 1-5 of every 28-day cycle for up to 6 cycles, starting 4 weeks after the end of RT. Patients in the control arm received RT only. Pneumocystis jirovecii pneumonia (PCP) prophylaxis was required during RT and combined TMZ therapy.
TMZ was administered as salvage therapy in the follow-up phase in 161 patients of the 282 (57%) in the RT alone arm, and 62 patients of the 277 (22%) in the TMZ + RT arm.
The hazard ratio (HR) for overall survival was 1.59 (95% CI for HR=1.33-1.91) with a log-rank p<0.0001 in favour of the TMZ arm. The estimated probability of surviving 2 years or more (26% vs 10%) is higher for the RT + TMZ arm. The addition of concomitant TMZ to RT, followed by TMZ monotherapy in the treatment of patients with newly-diagnosed glioblastoma multiforme demonstrated a statistically significant improvement in overall survival (OS) compared with RT alone (Figure 1).
Figure 1. Kaplan-Meier curves for overall survival (intent-to-treat population):
The results from the trial were not consistent in the subgroup of patients with a poor performance status (WHO PS=2, n=70), where overall survival and time to progression were similar in both arms. However, no unacceptable risks appear to be present in this patient group.
Recurrent or progressive malignant glioma
Data on clinical efficacy in patients with glioblastoma multiforme (Karnofsky performance status [KPS]≥70), progressive or recurrent after surgery and RT, were based on two clinical trials with oral TMZ. One was a non-comparative trial in 138 patients (29% received prior chemotherapy), and the other was a randomised active-controlled trial of TMZ vs procarbazine in a total of 225 patients (67% received prior treatment with nitrosourea based chemotherapy). In both trials, the primary endpoint was progression-free survival (PFS) defined by MRI scans or neurological worsening. In the non- comparative trial, the PFS at 6 months was 19%, the median progression-free survival was 2.1 months, and the median overall survival 5.4 months. The objective response rate (ORR) based on MRI scans was 8%.
In the randomised active-controlled trial, the PFS at 6 months was significantly greater for TMZ than for procarbazine (21% vs 8%, respectively – chi-square p=0.008) with median PFS of 2.89 and 1.88 months respectively (log rank p=0.0063). The median survival was 7.34 and 5.66 months for TMZ and procarbazine, respectively (log rank p=0.33). At 6 months, the fraction of surviving patients was significantly higher in the TMZ arm (60%) compared with the procarbazine arm (44%) (chi-square p=0.019). In patients with prior chemotherapy a benefit was indicated in those with a KPS 80.
Data on time to worsening of neurological status favoured TMZ over procarbazine as did data on time to worsening of performance status (decrease to a KPS of <70 or a decrease by at least 30 points). The median times to progression in these endpoints ranged from 0.7 to 2.1 months longer for TMZ than for procarbazine (log rank p= <0.01 to 0.03).
Recurrent anaplastic astrocytoma
In a multicentre, prospective phase II trial evaluating the safety and efficacy of oral TMZ in the treatment of patients with anaplastic astrocytoma at first relapse, the 6 month PFS was 46%. The median PFS was 5.4 months. Median overall survival was 14.6 months. Response rate, based on the central reviewer assessment, was 35% (13 CR and 43 PR) for the intent-to-treat population (ITT) n=162. In 43 patients stable disease was reported. The 6-month event-free survival for the ITT population was 44% with a median event-free survival of 4.6 months, which was similar to the results for the progression-free survival. For the eligible histology population, the efficacy results were similar. Achieving a radiological objective response or maintaining progression-free status was strongly associated with maintained or improved quality of life.
Paediatric population
Oral TMZ has been studied in paediatric patients (age 3-18 years) with recurrent brainstem glioma or recurrent high grade astrocytoma, in a regimen administered daily for 5 days every 28 days. Tolerance to TMZ is similar to adults.
Pharmacokinetic properties
TMZ is spontaneously hydrolyzed at physiologic pH primarily to the active species, 3-methyl(triazen-1-yl)imidazole-4-carboxamide (MTIC). MTIC is spontaneously hydrolyzed to 5amino-imidazole4carboxamide (AIC), a known intermediate in purine and nucleic acid biosynthesis, and to methylhydrazine, which is believed to be the active alkylating species. The cytotoxicity of MTIC is thought to be primarily due to alkylation of DNA mainly at the O6 and N7 positions of guanine. Relative to the AUC of TMZ, the exposure to MTIC and AIC is ~ 2.4% and 23%, respectively. In vivo, the t½ of MTIC was similar to that of TMZ, 1.8 hr.
In an open-label, two-way crossover bioequivalence study of the pharmacokinetics of oral and intravenous TMZ in patients with primary CNS malignancies, Temodal 2.5 mg/ml powder for solution for infusion administered over 90 minutes was found to be bioequivalent for Cmax and AUC of TMZ and MTIC as compared to Temodal hard capsules, following administration of 150 mg/m² dose. Mean Cmax values for TMZ and MTIC were 7.4 μg/ml and 320 ng/ml, respectively, following 90 minute intravenous infusion. Mean AUC(0→∞) values for TMZ and MTIC were 25 μg•h/ml and 1,004 ng•h/ml, respectively.
Absorption
After oral administration to adult patients, TMZ is absorbed rapidly, with peak concentrations reached as early as 20 minutes post-administration (mean time between 0.5 and 1.5 hours). After oral administration of 14C-labelled TMZ, mean faecal excretion of 14C over 7 days post-dose was 0.8% indicating complete absorption.
Distribution
TMZ demonstrates low protein binding (10% to 20%), and thus it is not expected to interact with highly protein-bound substances.
PET studies in humans and preclinical data suggest that TMZ crosses the blood-brain barrier rapidly and is present in the CSF. CSF penetration was confirmed in one patient; CSF exposure based on AUC of TMZ was approximately 30% of that in plasma, which is consistent with animal data.
Elimination
The half-life (t1/2) in plasma is approximately 1.8 hours. The major route of 14C elimination is renal. Following oral administration, approximately 5% to 10% of the dose is recovered unchanged in the urine over 24 hours, and the remainder excreted as temozolomide acid, 5-aminoimidazole-4-carboxamide (AIC) or unidentified polar metabolites.
Plasma concentrations increase in a dose-related manner. Plasma clearance, volume of distribution and half-life are independent of dose.
Special populations
Analysis of population-based pharmacokinetics of TMZ revealed that plasma TMZ clearance was independent of age, renal function or tobacco use. In a separate pharmacokinetic study, plasma pharmacokinetic profiles in patients with mild to moderate hepatic impairment were similar to those observed in patients with normal hepatic function.
Paediatric patients had a higher AUC than adult patients; however, the maximum tolerated dose (MTD) was 1,000 mg/m² per cycle both in children and in adults.
Preclinical safety data
Single-cycle (5-day dosing, 23 days non-treatment), 3- and 6-cycle toxicity studies were conducted in rats and dogs. The primary targets of toxicity included the bone marrow, lymphoreticular system, testes, the gastrointestinal tract and, at higher doses, which were lethal to 60% to 100% of rats and dogs tested, degeneration of the retina occurred. Most of the toxicity showed evidence of reversibility, except for adverse events on the male reproductive system and retinal degeneration. However, because the doses implicated in retinal degeneration were in the lethal dose range, and no comparable effect has been observed in clinical studies, this finding was not considered to have clinical relevance.
TMZ is an embryotoxic, teratogenic and genotoxic alkylating agent. TMZ is more toxic to the rat and dog than to humans, and the clinical dose approximates the minimum lethal dose in rats and dogs. Dose-related reductions in leukocytes and platelets appear to be sensitive indicators of toxicity. A variety of neoplasms, including mammary carcinomas, keratocanthoma of the skin and basal cell adenoma were observed in the 6-cycle rat study while no tumours or pre-neoplastic changes were evident in dog studies. Rats appear to be particularly sensitive to oncogenic effects of TMZ, with the occurrence of first tumours within 3 months of initiating dosing. This latency period is very short even for an alkylating agent.
Results of the Ames/salmonella and Human Peripheral Blood Lymphocyte (HPBL) chromosome aberration tests showed a positive mutagenicity response.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.