TEPEZZA Powder for solution for injection Ref.[10242] Active ingredients: Teprotumumab
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Teprotumumab-trbw’s mechanism of action in patients with Thyroid Eye Disease has not been fully characterized. Teprotumumab-trbw binds to IGF-1R and blocks its activation and signaling.
12.2. Pharmacodynamics
No formal pharmacodynamic studies have been conducted with teprotumumab-trbw.
12.3. Pharmacokinetics
The pharmacokinetics of teprotumumab-trbw was described by a two compartment population PK model based on data from 40 patients with Thyroid Eye Disease receiving an initial intravenous infusion of 10 mg/kg, followed by infusions of 20 mg/kg TEPEZZA every 3 weeks in two clinical trials. Following this regimen, the mean (± standard deviation) estimates for steady-state area under the concentration curve (AUC), peak (Cmax), and trough (Ctrough) concentrations of teprotumumab-trbw were 138 (± 34) mg∙hr/mL, 632 (± 139) mcg/mL, and 176 (± 56) mcg/mL, respectively.
Distribution
Following the recommended TEPEZZA dosing regimen, the population PK estimated mean (± standard deviation) for central and peripheral volume of distribution of teprotumumab-trbw were 3.26 (±0.87) L and 4.32 (± 0.67) L, respectively. The mean (± standard deviation) estimated inter-compartment clearance was 0.74 (± 0.16) L/day.
Elimination
Following the recommended TEPEZZA dosing regimen, the population PK estimated mean (± standard deviation) for the clearance of teprotumumab-trbw was 0.27 (± 0.08) L/day and for the elimination half-life was 20 (± 5) days.
Metabolism
Metabolism of teprotumumab-trbw has not been fully characterized. However, teprotumumab-trbw is expected to undergo metabolism via proteolysis.
Specific Populations
No clinically significant differences in the pharmacokinetics of teprotumumab-trbw were observed following administration of TEPEZZA based on patient’s age (18-80 years), gender, race/ethnicity (103 White, 10 Black, and 3 Asian), weight (46-169 kg), mild to moderate renal impairment (creatinine clearance 30 to 89 mL/min estimated by Cockcroft-Gault Equation), bilirubin levels (2.7-24.3 mcmol/L), aspartate aminotransferase (AST) levels (11-221 U/L), or alanine aminotransferase (ALT) levels (7-174 U/L). The effect of hepatic impairment on the pharmacokinetics of teprotumumab-trbw is unknown.
Drug Interactions
No studies evaluating the drug interaction potential of TEPEZZA have been conducted.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of TEPEZZA has not been evaluated in long-term animal studies.
Mutagenesis
The genotoxic potential of TEPEZZA has not been evaluated.
Impairment of Fertility
Fertility studies have not been performed with TEPEZZA.
14. Clinical Studies
TEPEZZA was evaluated in 2 randomized, double-masked, placebo-controlled studies in 171 patients with Thyroid Eye Disease: Study 1 (NCT01868997) and Study 2 (NCT03298867). Patients were randomized to receive TEPEZZA or placebo in a 1:1 ratio. Patients were given intravenous infusions (10 mg/kg for first infusion and 20 mg/kg for the remaining 7 infusions) every 3 weeks for a total of 8 infusions. Patients had a clinical diagnosis of Thyroid Eye Disease with symptoms and were euthyroid or had thyroxine and free triiodothyronine levels less than 50% above or below normal limits. Prior surgical treatment for Thyroid Eye Disease was not permitted. Proptosis ranged from 16 to 33 mm and 125 patients (73%) had diplopia at baseline.
A total of 84 patients were randomized to TEPEZZA and 87 patients were randomized to placebo. The median age was 52 years (range 20 to 79 years), 86% were White, 9% were Black or African-American, 4% were Asian and 1% identified as Other. The majority (73%) were female. At baseline, 27% of patients were smokers.
The proptosis responder rate at week 24 was defined as the percentage of patients with ≥2 mm reduction in proptosis in the study eye from baseline, without deterioration in the non-study eye (≥2 mm increase) in proptosis. Additional evaluations included signs and symptoms of Thyroid Eye Disease including pain, gaze evoked orbital pain, swelling, eyelid erythema, redness, chemosis, inflammation, clinical activity score and assessments of functional vision and patient appearance. Results for proptosis are found in Table 2.
Table 2. Efficacy Results in Patients with Thyroid Eye Disease in Study 1 and 2:
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
| Teprotumumab (N=42) | Placebo (N=45) | Difference (95% CI) | Teprotumumab (N=41) | Placebo (N=42) | Difference (95% CI) | |
| Proptosis responder rate at week 24, % (n)* | 71% (30) | 20% (9) | 51% (33, 69) | 83% (34) | 10% (4) | 73% (59, 88) |
| Proptosis (mm) average change from baseline through week 24, LS Mean (SE)† | -2.5 (0.2) | -0.2 (0.2) | -2.3 (-2.8, -1.8) | -2.8 (0.2) | -0.5 (0.2) | -2.3 (-2.8, -1.8) |
* Difference and its corresponding 95% Confidence Interval (CI) is based on a weighted average of the difference within each randomization stratum (tobacco user, tobacco nonuse) using CMH weights.
† Results were obtained from an MMRM with an unstructured covariance matrix and including treatment, smoking status, baseline value, visit, treatment by visit, and visit by baseline value interaction as fixed effects. A change from Baseline of 0 was imputed at the first postBaseline visit for any subject without a post-Baseline value.
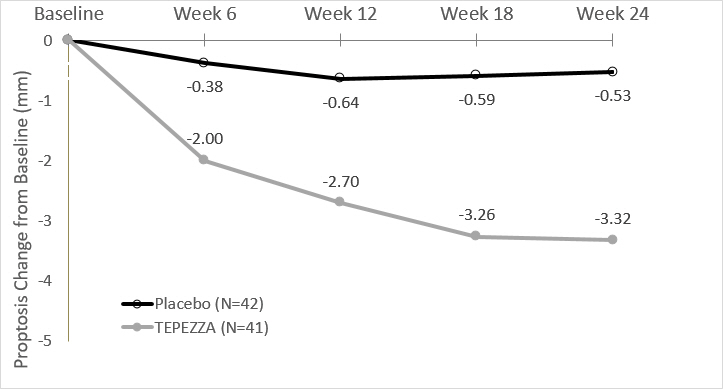
In Study 2, improvement of proptosis as measured by mean change from Baseline was observed as early as 6 weeks and continued to improve through week 24 as shown in Figure 1. Similar results were seen in Study 1.
Figure 1. Change from Baseline in Proptosis over 24 Weeks in Study 2
P<0.01 at each timepoint
TEPEZZA also led to improvement in the less severely impacted “fellow” eye.
Diplopia (double vision) was evaluated in a subgroup of patients that had diplopia at baseline in Study 1 and 2. Results are shown in Table 3.
Table 3. Diplopia in Patients with Thyroid Eye Disease in Study 1 and 2:
| Parameter | TEPEZZA (n=66) | Placebo (n=59) |
|---|---|---|
| Diplopia | ||
| Responder rate* at week 24, % (n) | 53% (35) | 25% (15) |
P<0.01
* Diplopia was evaluated on a 4 -point scale where scores ranged from 0 for no diplopia to 3 for constant diplopia. A diplopia responder was defined as a patient with baseline diplopia >0 and a score of 0 at week 24.
Following discontinuation of treatment in Study 1, 53% of patients (16 of 30 patients) who were proptosis responders at week 24 maintained proptosis response 51 weeks after the last TEPEZZA infusion. 67% of patients (12 of 18) who were diplopia responders at week 24 maintained diplopia response 51 weeks after the last TEPEZZA infusion.
Subgroups
Examination of age and gender subgroups did not identify differences in response to TEPEZZA among these subgroups. Reduction in proptosis was similar between smokers and non-smokers in both studies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.