TESTAVAN Transdermal gel Ref.[27746] Active ingredients: Testosterone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Ferring Pharmaceuticals Ltd, Drayton Hall, Church Road, West Drayton, UB7 7PS, UK
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Androgens
ATC code: G03BA03
Testosterone and dihydrotestosterone (DHT), endogenous androgens, are responsible for the normal growth and development of the male sex organs and for the maintenance of secondary sex characteristics. These effects include the growth and maturation of the prostate, seminal vesicles, penis and scrotum; the development of male hair distribution on the face, chest, axillae and pubis; laryngeal enlargement, vocal chord thickening, alterations in body musculature and fat distribution.
Insufficient secretion of testosterone due to testicular failure, pituitary pathology or gonadotropin or luteinising hormone-releasing hormone deficiency results in male hypogonadism and low serum testosterone concentration. Symptoms associated with low testosterone include decreased sexual desire with or without impotence, fatigue, loss of muscle mass, mood depression and regression of secondary sexual characteristics.
Restoring testosterone levels to within the normal range can result in improvements over time in muscle mass, mood, sexual desire, libido and sexual function including sexual performance and number of spontaneous erections.
During exogenous administration of testosterone to normal males, endogenous testosterone release may be decreased through feedback inhibition of pituitary luteinising hormone (LH). With large doses of exogenous androgens, spermatogenesis may also be suppressed through inhibition of pituitary follicle stimulating hormone (FSH).
Androgen administration causes retention of sodium, nitrogen, potassium, phosphorus and decreased urinary excretion of calcium. Androgens have been reported to increase protein anabolism and decrease protein catabolism. The nitrogen balance is improved only with sufficient intake of calories and protein. Androgens have been reported to stimulate production of red blood cells by enhancing the production of erythropoietin.
5.2. Pharmacokinetic properties
Testavan delivers physiologic amounts of testosterone, which provide a level of circulating testosterone similar to the normal level in healthy men (i.e., 300-1050 ng/dL). Testavan was evaluated in a multi-center, open-label, 120 day Phase 3 clinical study (study 000127) in 159 hypogonadal men ages 18 to 75 years (mean age 54.1 years). Subjects were white (77%), black (20%), Asian (2%), and multiracial (1%). In the phase 3 study, at the end of a 90 day treatment period during which the dose of Testavan could be titrated based on total testosterone concentrations, 76.1% of men had average testosterone concentrations over a 24 hour period (Cave) within the eugonadal range (300–1050 ng/dL).
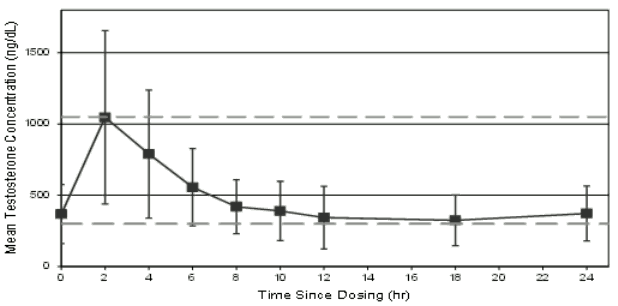
The mean testosterone concentration profile on Day 90 is shown in Figure 1, while the pharmacokinetic parameters for total testosterone on Day 90 are summarised for each Testavan dose in Table 11.
Figure 1. Mean ±SD serum concentrations of testosterone on day 90 after dose titration of Testavan:
Table 1. Pharmacokinetic parameters for total testosterone on day 90 after titration, study 000127 full analysis set:
| Testavan Dose on Day 90 | N | Cmin (ng/dL) Mean ±SD | Cave (ng/dL) Mean ±SD | Cmax (ng/dL) Mean ±SD | Tmax (hr) Median |
|---|---|---|---|---|---|
| 23 mg | 5 | 191 ± 49 | 368 ± 121 | 721 ± 254 | 4.02 |
| 46 mg | 45 | 277 ± 140 | 506 ± 207 | 1,228 ± 640 | 2.02 |
| 69 mg | 89 | 229 ± 82 | 438 ± 164 | 1,099 ± 595 | 2.08 |
Cmin: minimum concentration; Cave: average concentration over a 24 hour period; Cmax: maximum concentration; Tmax: time of maximum concentration; SD: standard deviation
Absorption
Testavan provides transdermal delivery of testosterone, with a median Tmax of approximately 2-4 hours after dosing. Total testosterone concentrations return to pre-dose values approximately 12 hours after application and no accumulation occurs after daily application for 10 days. Application on the upper arm and shoulder results in higher serum testosterone concentrations compared to application on the abdomen or the inner side of the thigh. The mean Cmax was 926, 451 and 519 ng/dL, respectively, and the mean Cave 557, 372 and 395 ng/dL, respectively.
Phase 2 study results show that total testosterone concentrations increased with increasing dose after daily application of 23, 46 and 69 mg Testavan.
Distribution
Circulating testosterone is chiefly bound in the serum to sex hormone-binding globulin (SHBG) and albumin. The albumin-bound fraction of testosterone easily dissociates from albumin and is presumed to be biologically active. The portion of testosterone bound to SHBG is not considered biologically active. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins.
Biotransformation
There is considerable variation in the half-life of testosterone, as reported in the literature, ranging from ten to 100 minutes.
Testosterone is metabolised to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are oestradiol and dihydrotestosterone (DHT).
Elimination
About 90% of testosterone given intramuscularly is excreted in the urine as glucuronic and sulphuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the faeces, mostly in the unconjugated form.
Effect of Showering
Showering 1 hour and 2 hours following Testavan administration decreased Cave by 19.2% and 14.3%, respectively, compared with subjects who did not shower after Testavan administration. Showering 6 hours following Testavan administration did not result in a decrease in Cave.
Renal function
Testosterone Cave and Cmax was similar in subjects with normal renal function and subjects with mild and moderate renal impairment. No data is available in subjects with severe renal impairment.
5.3. Preclinical safety data
Toxicological studies have not revealed other effects than those which can be explained on the base of the hormone profile of Testavan.
Fertility studies in rodents and primates have shown that treatment with testosterone can impair male fertility by suppressing spermatogenesis in a dose dependent manner.
Testosterone has been found to be non-mutagenic in vitro using the reverse mutation model (Ames test) or Chinese hamster ovary cell line. A relationship between androgen treatment and certain cancer forms has been found in laboratory animals. Data in rats have shown increased incidences of prostate cancer after treatment with testosterone.
Sex hormones are known to facilitate the development of certain types of tumour induced by known carcinogenic agents. No correlation between these findings and the actual risk in human beings has been established.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.