TETRACYCLINE HYDROCHLORIDE Capsule Ref.[107312] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
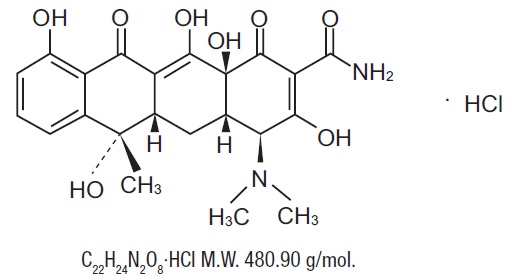
Tetracycline, USP is a yellow, odorless, crystalline powder. Tetracycline, USP is stable in air but exposure to strong sunlight causes it to darken. Its potency is affected in solutions of pH below 2 and is rapidly destroyed by alkali hydroxide solutions. Tetracycline, USP is very slightly soluble in water, freely soluble in dilute acid and in alkali hydroxide solutions, sparingly soluble in alcohol, and practically insoluble in chloroform and in ether. The chemical name for tetracycline hydrochloride, USP is (4S,4aS,5aS,6S,12aS)4(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,-12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride.
Its structural formula is as follows:
Each capsule, for oral administration, contains 250 mg or 500 mg tetracycline hydrochloride, USP.
Inactive Ingredients: Colloidal silicon dioxide, pregelatinized starch (corn), and stearic acid.
The 250 mg and 500 mg capsule shells contain D&C yellow #10, FD&C blue #1, FD&C yellow #6, gelatin, and titanium dioxide.
The imprinting ink for the 250 mg and 500 mg capsules contains D&C yellow #10, ethanol, FD&C blue #1, FD&C blue #2, FD&C red #40, iron oxide black, methanol, n-butyl alcohol, propylene glycol and shellac glaze.
USP Dissolution Test 2.
| How Supplied |
|---|
|
Tetracycline hydrochloride capsules, USP, 250 mg, are supplied as hard gelatin capsules with light blue opaque cap and yellow opaque body printed AMNEAL on cap and 1522 on body with black ink. They are available as follows: Bottles of 100: NDC 60219-1522-1 Tetracycline hydrochloride capsules, USP, 500 mg, are supplied as hard gelatin capsules with light blue opaque cap and yellow opaque body printed AMNEAL on cap and 1523 on body with black ink. They are available as follows: Bottles of 100: NDC 60219-1523-1 Dispense in a tight, light-resistant container as defined in the USP. Use child-resistant closure (as required). Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. Manufactured by: Amneal Pharmaceuticals Pvt. Ltd., Oral Solid Dosage Unit, Ahmedabad 382213, INDIA Distributed by: Amneal Pharmaceuticals LLC, Bridgewater, NJ 08807 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.