THAMICARB Oral solution Ref.[49927] Active ingredients: Sodium bicarbonate
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Syri Limited, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 0NU, UK Trading as: Thame Laboratories, Unit 4, Bradfield Road, Ruislip, Middlesex, HA4 0NU, UK. OR Trading as: SyriMed, Unit 4, Bradfield ...
4.1. Therapeutic indications
Thamicarb is used to treat hyperacidity, dyspepsia and symptomatic relief of heartburn and peptic ulceration.
Thamicarb is also indicated for the treatment of metabolic acidosis in adults with chronic kidney disease.
4.2. Posology and method of administration
Posology
For acid indigestion
Adults and children over 12 years
Take 12ml (1g) to 60ml (5g) every 4 to 6 hours.
Not recommended for use in children under 12 years of age.
For metabolic acidosis in chronic kidney disease
Adults (including elderly)
Metabolic Acidosis: Dosage is calculated on an individual basis and administered according to the acid-base balance and electrolyte status.
Children
There is no experience using Thamicarb in the management of metabolic acidosis in children.
Method of administration
The required dose should be drawn from the container into the graduated syringe using the syringe adaptor (see section 6.6).
4.9. Overdose
Excessive administration of bicarbonate may lead to hypokalaemia and metabolic alkalosis, especially in patients with impaired renal function. Symptoms include mood changes, tiredness, shortness of breath, muscle weakness and irregular heart beat. Muscle hypertonicity, twitching and tetany may develop, especially in hypocalcaemic patients. Excessive doses of sodium salts may lead to sodium overloading and hyperosmolality.
Treatment of metabolic alkalosis and hypernatraemia is by correction of fluid and electrolyte balance. Replacement of calcium, chloride, and potassium ions may be of particular importance.
6.3. Shelf life
12 months.
For 100ml bottle: Discard your medicine 3 days after first opening.
For 500ml bottle: Discard your medicine 7 days after first opening.
6.4. Special precautions for storage
Do not store above 25°C.
Do not refrigerate or freeze.
Do not use if crystals are observed in the product.
For storage conditions after first opening of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Bottle: Amber glass.
Closure: White tamper-evident child-resistant polypropylene cap with HDPE-EPE wadding.
Pack size: 100ml or 500ml.
Dosing Device: 20ml white polypropylene oral syringe with 1ml graduation marks and LDPE syringe adaptor.
6.6. Special precautions for disposal and other handling
The required dose should be drawn from the container into the graduated syringe provided using the syringe adaptor (see detailed instructions below). The syringe should be held into the mouth of the patient, and the contents of the syringe should then be ejected into the mouth and swallowed.
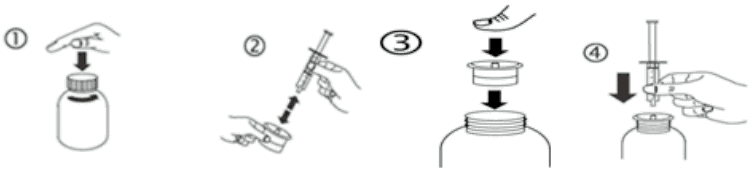
Instructions for the use of syringe:
a) Open the bottle: press the cap and turn it anticlockwise (figure 1).
b) Separate the adaptor from the syringe (figure 2). Insert the adaptor into the bottle neck (figure 3). Ensure it is properly fixed. Take the syringe and put it in the adaptor opening (figure 4).
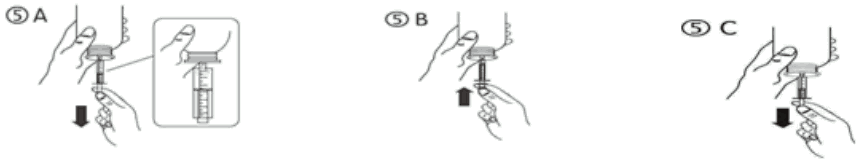
c) Turn the bottle upside down. Fill the syringe with a small amount of solution by pulling the piston down (figure 5A), then push the piston upwards in order to remove any possible bubble (figure 5B). Pull the piston down to the graduation mark corresponding to the quantity in millilitres (ml) prescribed by your doctor (figure 5C).
d) Turn the bottle the right way up (figure 6A). Remove the syringe from the adaptor (figure 6B).
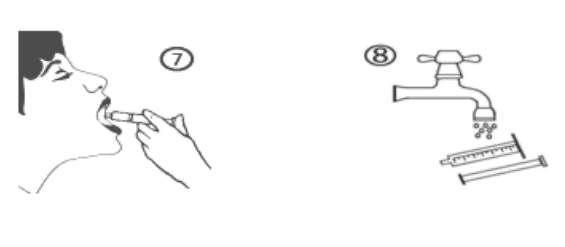
e) Empty the contents of the syringe into the patient’s mouth by pushing the piston to the bottom of the syringe (figure 7). The contents of the syringe should be emptied into the side cheek of the patients mouth to avoid a choking hazard. Close the bottle with the plastic screw cap. Wash the syringe with water (figure 8).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.