TIMOPTIC Ophthalmic solution Ref.[11094] Active ingredients: Timolol
Source: FDA, National Drug Code (US) Revision Year: 2011
Product description
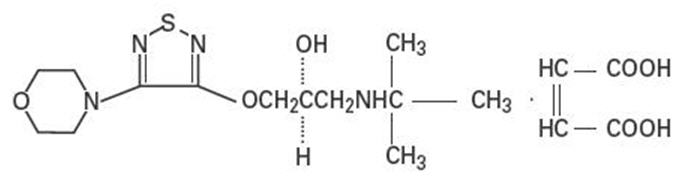
Timolol maleate is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)1(tert-butylamino)3[(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is:
| [α] | 25° 405 nm | in 1.0N HCl (C = 5%) = –12.2° (–11.7° to –12.5°). |
Its molecular formula is C13H24N4O3S•C4H4O4 and its structural formula is:
Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol. Timolol maleate is stable at room temperature.
Timolol maleate ophthalmic solution is supplied in two formulations: Ophthalmic Solution TIMOPTIC (timolol maleate ophthalmic solution), which contains the preservative benzalkonium chloride; and Ophthalmic Solution TIMOPTIC (timolol maleate ophthalmic solution), the preservative-free formulation.
Preservative-free Ophthalmic Solution TIMOPTIC is supplied in OCUDOSE, a unit dose container, as a sterile, isotonic, buffered, aqueous solution of timolol maleate in two dosage strengths: Each mL of Preservative-free TIMOPTIC in OCUDOSE 0.25% contains 2.5 mg of timolol (3.4 mg of timolol maleate). The pH of the solution is approximately 7.0, and the osmolarity is 252-328 mOsm. Each mL of Preservative-free TIMOPTIC in OCUDOSE 0.5% contains 5 mg of timolol (6.8 mg of timolol maleate).
Inactive ingredients: monobasic and dibasic sodium phosphate, sodium hydroxide to adjust pH, and water for injection.
| How Supplied |
|---|
|
Preservative-free Sterile Ophthalmic Solution TIMOPTIC in OCUDOSE is a clear, colorless to light yellow solution. No. 814 – Preservative-free TIMOPTIC, 0.25% timolol equivalent, is supplied in OCUDOSE, a clear low density polyethylene unit dose container. Each individual unit contains 0.2 mL of solution, and is available in a foil laminate overwrapped pouch as follows: NDC 25010-814-66; 60 Individual Unit Doses. No. 815 – Preservative-free TIMOPTIC, 0.5% timolol equivalent, is supplied in OCUDOSE, a clear low density polyethylene unit dose container. Each individual unit contains 0.2 mL of solution, and is available in a foil laminate overwrapped pouch as follows: NDC 25010-815-66; 60 Individual Unit Doses. Manuf. for: ATON PHARMA, Lawrenceville, NJ 08648, USA By: Laboratories Merck Sharp & Dohme-Chibret, 63963 Clermont-Ferrand Cedex 9, France |
Drugs
| Drug | Countries | |
|---|---|---|
| TIMOPTIC | Austria, Canada, Poland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.