TIROSINT Capsule Ref.[50795] Active ingredients: Levothyroxine
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
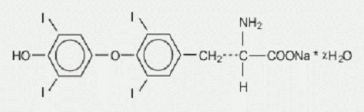
TIROSINT (levothyroxine sodium) capsules for oral use contain synthetic L-3,3',5,5'- tetraiodothyronine sodium salt [levothyroxine (T4) sodium]. Synthetic T4 is chemically identical to that produced in the human thyroid gland. Levothyroxine (T4) sodium has an empirical formula of C15H10I4NNaO4 ∙ x H2O (where x = 5), molecular weight of 798.86 g/mol (anhydrous), and structural formula as shown:
TIROSINT (levothyroxine sodium) capsules are amber-colored, round/biconvex capsules containing a viscous amber-colored liquid.
The inactive ingredients in TIROSINT are gelatin, glycerin and water.
| Dosage Forms and Strengths | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TIROSINT capsules are amber-colored, round/biconvex capsules, imprinted with a
|
| How Supplied | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
TIROSINT (levothyroxine sodium) capsules are amber-colored, round/biconvex capsules, imprinted with a dosage strength specific letter on one side and containing a viscous amber-colored liquid. They are supplied as follows: Table 7. TIROSINT Packaging Description – Boxes of 30 capsules, consisting of 3 blisters with 10 capsules each:
* Shown on box and blister packing, not on individual capsules. The dosage strength on each box is clearly identified in several locations, and is associated with a distinct color. The color of the circles on the blister is the same color as on the box. Each blister pack contains 10 capsules placed in individual cavities labeled with the dosage strength and the product name (TIROSINT). Manufactured for IBSA Pharma Inc. by: IBSA Institut Biochimique SA, 6912 Pazzallo, Switzerland Distributed by: IBSA Pharma Inc., Parsippany, NJ 07054, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| TIROSINT | Netherlands, Poland, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.