TRANXENE Tablet Ref.[50790] Active ingredients: Clorazepate
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
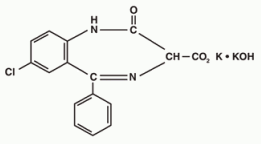
Chemically, TRANXENE is a benzodiazepine. The empirical formula is C16H11ClK2N2O4; the molecular weight is 408.92; 1H-1, 4 Benzodiazepine-3-carboxylic acid, 7-chloro-2, 3-dihydro-2-oxo-5-phenyl-, potassium salt compound with potassium hydroxide (1:1) and the structural formula may be represented as follows:
The compound occurs as a fine, light yellow, practically odorless powder. It is insoluble in the common organic solvents, but very soluble in water. Aqueous solutions are unstable, clear, light yellow, and alkaline.
Each tablet contains 7.5 mg of Clorazepate Dipotassium, USP equivalent to 5.8 mg of Clorazepate.
Inactive ingredients for TRANXENE T-TAB Tablets: Colloidal silicon dioxide, FD&C Yellow No. 6, magnesium oxide, magnesium stearate, microcrystalline cellulose, potassium carbonate, potassium chloride, and talc.
| How Supplied |
|---|
|
TRANXENE 7.5 mg scored T-TAB tablets are supplied as peach-colored tablets bearing the letter R, the distinctive T shape and a two-digit designation, 32: Bottles of 100 (NDC 55292-304-01). T-TAB tablet appearance and shape are registered trademarks of Lundbeck LLC. Manufactured by: UPM Pharmaceuticals, 510 5th Street, Bristol, TN 37620, U.S.A. For: Recordati Rare Diseases Inc., Lebanon, NJ 08833, U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| TRANXENE | France, Lithuania, Netherlands, Poland, Tunisia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.