TREANDA Solution for injection Ref.[11064] Active ingredients: Bendamustine
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
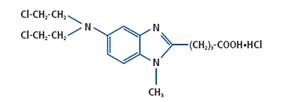
TREANDA (bendamustine hydrochloride) is an alkylating agent. The chemical name of bendamustine hydrochloride is 1H-benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]1 methyl, monohydrochloride. Its empirical molecular formula is C16H21Cl2N3O2∙HCl, and the molecular weight is 394.7.
Bendamustine hydrochloride contains a mechlorethamine group and a benzimidazole heterocyclic ring with a butyric acid substituent, and has the following structural formula:
TREANDA Injection (45 mg/0.5 mL or 180 mg/2 mL solution)
TREANDA (bendamustine HCl) Injection for intravenous use is supplied as a sterile clear colorless to yellow solution in a single-dose vialEach 0.5 mL vial contains 45 mg of bendamustine hydrochloride, 162 mg of Propylene Glycol, USP and 293 mg of N,N-Dimethylacetamide, EP. Each 2 mL vial contains 180 mg of bendamustine hydrochloride, 648 mg of Propylene Glycol, USP and 1172 mg of N,N-Dimethylacetamide, EP. An overfill of 0.2 mL is included in each vial.
TREANDA for Injection (25 mg/vial or 100 mg/vial lyophilized powder)
TREANDA (bendamustine HCl) for Injection for intravenous use is supplied as a sterile non-pyrogenic white to off-white lyophilized powder in a single-dose vial. Each 25-mg vial contains 25 mg of bendamustine hydrochloride and 42.5 mg of mannitol, USP. Each 100-mg vial contains 100 mg of bendamustine hydrochloride and 170 mg of mannitol, USP. The pH of the reconstituted solution is 2.5-3.5.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
Safe Handling and DisposalTREANDA (bendamustine hydrochloride) is a cytotoxic drug. Follow applicable special handling and disposal procedures1. Care should be exercised in the handling and preparation of solutions prepared from TREANDA Injection and TREANDA for Injection. The use of gloves and safety glasses is recommended to avoid exposure in case of breakage of the vial or other accidental spillage. If gloves come in contact with TREANDA Injection prior to dilution, remove gloves and follow disposal procedures1. If a solution of TREANDA (bendamustine hydrochloride) contacts the skin, wash the skin immediately and thoroughly with soap and water. If TREANDA (bendamustine hydrochloride) contacts the mucous membranes, flush thoroughly with water. How SuppliedTREANDA (bendamustine hydrochloride) Injection is supplied as a 90 mg/mL clear colorless to yellow solution in individual cartons as follows:
TREANDA (bendamustine hydrochloride) for Injection is supplied in individual cartons as follows:
Distributed By: Teva Pharmaceuticals USA, Inc., North Wales, PA 19454 |
Drugs
| Drug | Countries | |
|---|---|---|
| TREANDA | Canada, Hong Kong, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.