TRESIBA Solution for injection Ref.[107042] Active ingredients: Insulin degludec
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
Insulin degludec is a long-acting basal human insulin analog for subcutaneous injection produced by a process that includes expression of recombinant DNA in Saccharomyces cerevisiae followed by chemical modification.
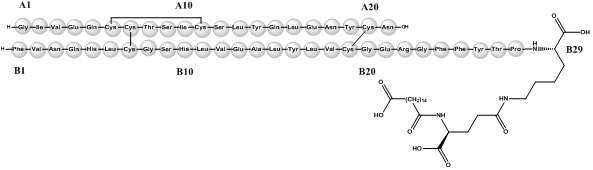
Insulin degludec differs from human insulin in that the amino acid threonine in position B30 has been omitted and a side-chain consisting of glutamic acid and a C16 fatty acid has been attached (chemical name: LysB29(Nε-hexadecandioyl-γ-Glu) des(B30) human insulin). Insulin degludec has a molecular formula of C274H411N65O81S6 and a molecular weight of 6.104 kDa. It has the following structure:
Figure 1. Structural Formula of Insulin Degludec:
TRESIBA (insulin degludec) injection is a sterile, aqueous, clear, and colorless solution available as 100 units/mL (U-100) or 200 units/mL (U-200) for subcutaneous use.
For the 100 units/mL solution, each mL contains 100 units of insulin degludec and glycerin (19.6 mg), metacresol (1.72 mg), phenol (1.5 mg), zinc (32.7 mcg), and Water for Injection, USP.
For the 200 units/mL solution, each mL contains 200 units of insulin degludec and glycerin (19.6 mg), metacresol (1.72 mg), phenol (1.5 mg), zinc (71.9 mcg), and Water for Injection, USP.
TRESIBA has a pH of approximately 7.6. Hydrochloric acid or sodium hydroxide may be added to adjust pH.
| Dosage Forms and Strengths |
|---|
|
Injection: Available as a clear and colorless solution:
|
| How Supplied |
|---|
|
Product: 50090-3491 NDC: 50090-3491-0 3 mL in a SYRINGE, PLASTIC / 3 in a CARTON Manufactured by: Novo Nordisk Inc., 800 Scudders Mill Road, Plainsboro, NJ 08536 |
Drugs
| Drug | Countries | |
|---|---|---|
| TRESIBA | Austria, Brazil, Canada, Cyprus, Ecuador, Estonia, Spain, Finland, France, Hong Kong, Croatia, Ireland, Italy, Japan, Lithuania, Nigeria, Netherlands, Poland, Romania, Singapore, Tunisia, Turkey, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.