TRIAMTERENE AND HYDROCHLOROTHIAZIDE Capsule Ref.[107987] Active ingredients: Hydrochlorothiazide and Triamterene
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
Each triamterene and hydrochlorothiazide capsule, USP for oral use contains triamterene, USP 37.5 mg and hydrochlorothiazide, USP 25 mg. Hydrochlorothiazide, USP is a diuretic/antihypertensive agent and triamterene, USP is an antikaliuretic agent.
Hydrochlorothiazide, USP is slightly soluble in water. It is soluble in dilute ammonia, dilute aqueous sodium hydroxide, and dimethylformamide. It is sparingly soluble in methanol.
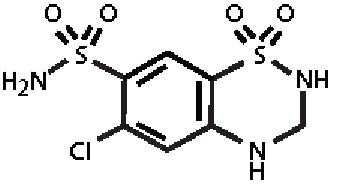
Hydrochlorothiazide, USP is 6-chloro-3,4-dihydro-2 H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide, and its structural formula is:
Molecular Formula: C 7H 8CIN 3O 4S 2 M.W. 297.74
At 50°C, triamterene, USP is practically insoluble in water (less than 0.1%). It is soluble in formic acid, sparingly soluble in methoxyethanol, and very slightly soluble in alcohol.
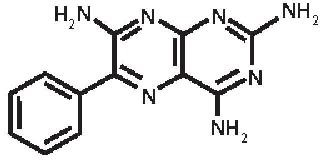
Triamterene, USP is 2,4,7-triamino-6-phenylpteridine and its structural formula is:
Molecular Formula: C 12H 11N 7 M.W. 253.26
Inactive ingredients consist of lactose monohydrate, pregelatinized starch, sodium starch glycolate, polysorbate 80, citric acid anhydrous, povidone, and magnesium stearate. The capsule shell consists of titanium dioxide and gelatin. The capsule imprinting ink consists of shellac glaze in ethanol, iron oxide black, n-butyl alcohol, propylene glycol, ethanol, methanol, FD&C Blue # 2 Aluminum Lake, FD&C Red # 40 Aluminum Lake, FD&C Blue # 1 Aluminum Lake, and D&C Yellow # 10 Aluminum Lake.
Triamterene and hydrochlorothiazide capsules, USP meet USP Dissolution Test 3 as published in the current USP monograph for Triamterene and Hydrochlorothiazide Capsules.
| How Supplied |
|---|
|
Triamterene and Hydrochlorothiazide Capsules, USP contain 37.5 mg triamterene, USP and 25 mg hydrochlorothiazide, USP and are available in size # 4 white opaque/white opaque capsules imprinted with Logo “LANNETT” on the cap and “1632” on the body. They are supplied as follows: Bottles of 90, NDC 62135-529-90 Manufactured for: Chartwell RX, LLC., Congers, NY 10920 |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.