TRICOR Tablet Ref.[50364] Active ingredients: Fenofibrate
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
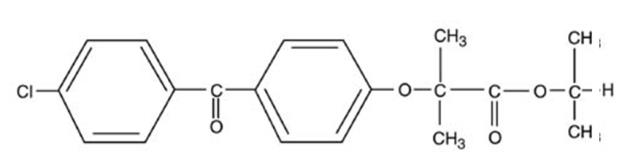
TRICOR (fenofibrate tablets), is a lipid regulating agent available as tablets for oral administration. Each tablet contains 48 mg or 145 mg of fenofibrate. The chemical name for fenofibrate is 2-[4-(4-chlorobenzoyl) phenoxy]-2-methyl-propanoic acid, 1-methylethyl ester with the following structural formula:
The empirical formula is C20H21O4Cl and the molecular weight is 360.83; fenofibrate is insoluble in water. The melting point is 79-82°C. Fenofibrate is a white solid which is stable under ordinary conditions.
Inactive Ingredients:
Each tablet contains hypromellose 2910 (3 cps), docusate sodium, sucrose, sodium lauryl sulfate, lactose monohydrate, silicified microcrystalline cellulose, crospovidone, and magnesium stearate.
In addition, individual tablets contain:
48 mg tablets: polyvinyl alcohol, titanium dioxide, talc, soybean lecithin, xanthan gum, D&C Yellow #10 aluminum lake, FD&C Yellow #6 /sunset yellow FCF aluminum lake, FD&C Blue #2 /indigo carmine aluminum lake.
145 mg tablets: polyvinyl alcohol, titanium dioxide, talc, soybean lecithin, xanthan gum.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
TRICOR (fenofibrate tablets) is available in two strengths: 48 mg: Yellow tablets, imprinted with the code identification letters “FI”, available in bottles of 90 (NDC 0074-3173-90). Yellow tablets, imprinted with the “a” logo and code identification letters “FI”, available in bottles of 90 (NDC 0074-6122-90). 145 mg: White tablets, imprinted with the code identification letters “FO”, available in bottles of 90 (NDC 0074-3189-90). White tablets, imprinted with the “a” logo and code identification letters “FO”, available in bottles of 90 (NDC 0074-6123-90). Manufactured for AbbVie Inc., North Chicago, IL 60064, U.S.A. by Fournier Laboratories Ireland Limited, Anngrove, Carrigtwohill Co. Cork, Ireland. |
Drugs
| Drug | Countries | |
|---|---|---|
| TRICOR | Croatia, Japan, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.