TRODELVY Powder for solution for infusion Ref.[28090] Active ingredients: Sacituzumab govitecan
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Gilead Sciences Ireland UC, Carrigtohill, County Cork, T45 DP77, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: antineoplastic agents, monoclonal antibodies and antibody drug conjugates, other monoclonal antibodies
ATC code: L01FX17
Mechanism of action
Sacituzumab govitecan binds to Trop-2-expressing cancer cells and is internalised with the subsequent release of SN-38 from a hydrolysable linker. SN-38 interacts with topoisomerase I and prevents re-ligation of topoisomerase I-induced single strand breaks. The resulting DNA damage leads to apoptosis and cell death.
Clinical efficacy and safety
Unresectable or metastatic Triple Negative Breast Cancer (ASCENT)
The efficacy and safety of sacituzumab govitecan was assessed in ASCENT (IMMU-132-05), an international Phase 3, multicentre, open-label, randomised study conducted in 529 patients with unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC) who had relapsed after at least two prior chemotherapies (no upper limit) for breast cancer. Earlier adjuvant or neoadjuvant therapy for more limited disease qualified as one of the required prior regimens if the development of unresectable, locally advanced or metastatic disease occurred within a 12-month period of time after completion of chemotherapy. All patients received previous taxane treatment in either the adjuvant, neoadjuvant, or advanced stage unless they had a contraindication or were intolerant to taxanes. Poly-ADP ribose polymerase (PARP) inhibitors were allowed as one of the two prior chemotherapies for patients with a documented germline BRCA1/BRCA2 mutation.
Patients were randomised (1:1) to receive sacituzumab govitecan 10 mg/kg as an intravenous infusion on Day 1 and Day 8 of a 21-day treatment cycle or Treatment of Physician's Choice (TPC) which was dosed based on body surface area and per the approved product information. TPC was determined by the investigator before randomisation from one of the following single-agent regimens: eribulin (n=139), capecitabine (n=33), gemcitabine (n=38), or vinorelbine (except if patient had ≥ Grade 2 neuropathy, n=52). Patients with stable brain metastases (pre-treated, non-progressive, without antiseizure medicinal products and on stable corticosteroid dose for at least 2 weeks) were eligible. Magnetic resonance imaging (MRI) to determine brain metastases was required only for patients with known or suspected brain metastases. Patients with known Gilbert's disease, bone-only disease, known history of unstable angina, myocardial infarction, or congestive heart failure, active chronic inflammatory bowel disease or gastrointestinal (GI) perforation, human immunodeficiency virus (HIV), active hepatitis B or C infection, live vaccine within 30 days, or who have previously received irinotecan were excluded.
Patients were treated until disease progression or unacceptable toxicity. The primary efficacy endpoint was progression-free survival (PFS) in patients without brain metastases at baseline (i.e. BMNeg) as measured by a blinded, independent, centralised review (BICR) group of radiology experts using Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 criteria. Secondary efficacy endpoints included PFS by BICR for the overall population, including all patients with and without brain metastases, overall survival (OS), objective response rate (ORR) and duration of response (DOR).
The primary analysis included 235 BMNeg patients in the sacituzumab govitecan group and 233 BMNeg patients in the TPC group. The analysis of the overall population included 267 patients in the sacituzumab govitecan group and 262 patients in the TPC group.
The demographics and baseline characteristics of the overall population (n=529) were: median age of 54 years (range: 27–82 years) and 81% <65 years; 99.6% female; 79% White; 12% Black; median number of prior systemic therapies was 4; 69% had previously received 2 to 3 prior chemotherapies; 31% had previously received >3 prior chemotherapies; 42% had hepatic metastases; 12% had present or a history of brain metastases. 8% were BRCA1/BRCA2 mutational status positive; BRCA status was available for 339 patients. At study entry, all patients had an ECOG performance status of 0 (43%) or 1 (57%). The median time from diagnosis of Stage 4 to study entry was 16.2 months (range: -0.4 to 202.9 months). The most frequent prior chemotherapies were cyclophosphamide (83%), anthracycline (83%) including doxorubicin (53%), paclitaxel (78%), carboplatin (65%), capecitabine (67%), gemcitabine (36%), docetaxel (35%), and eribulin (33%). Overall, 29% of patients had received prior PD-1/PD-L1 therapy. Thirteen percent of patients in the sacituzumab govitecan group in the overall population received only 1 prior line of systemic therapy in the metastatic setting.
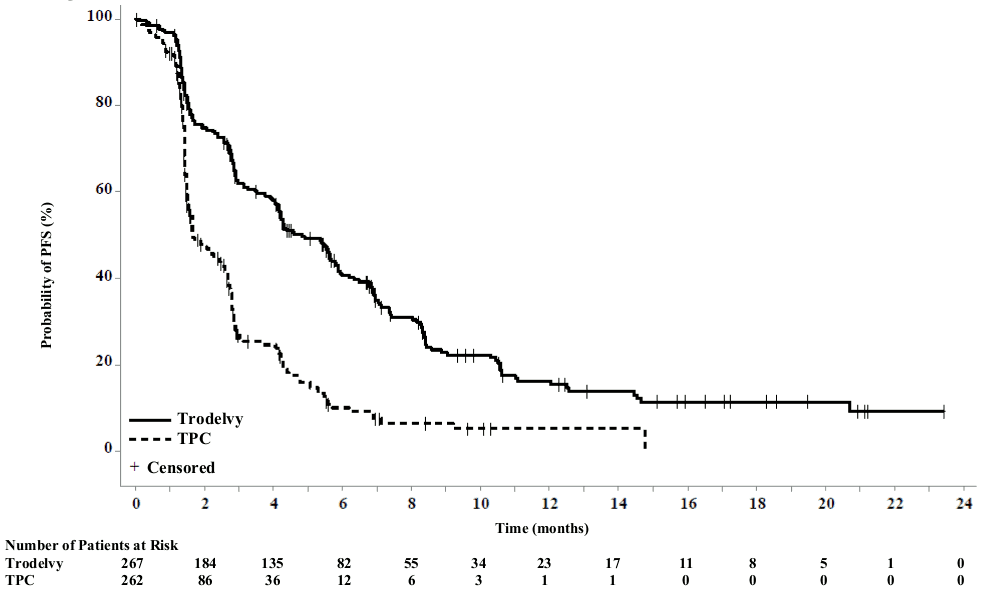
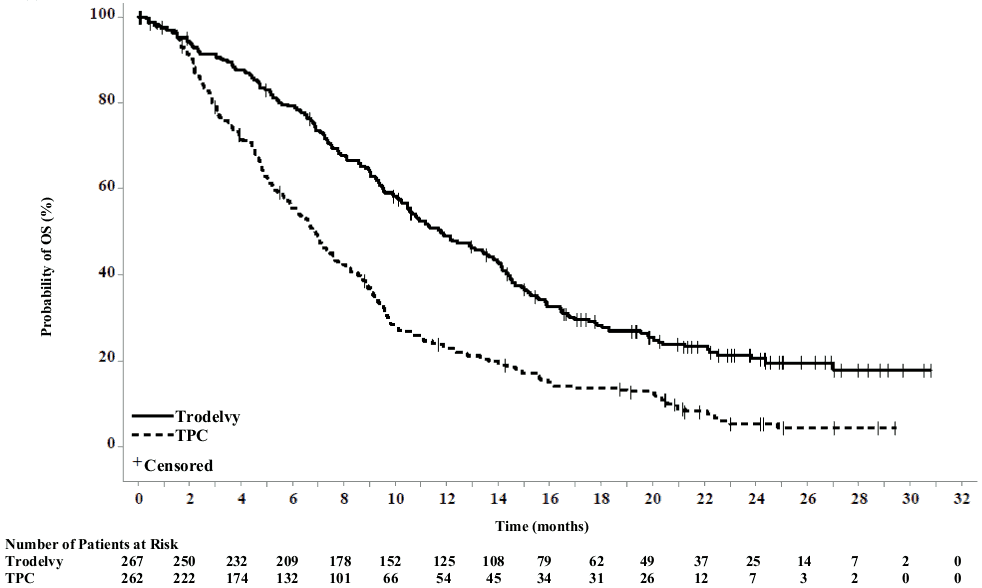
The efficacy results in the BMNeg population showed a statistical significant improvement of sacituzumab govitecan over TPC in PFS and OS with hazard ratios (HR) of 0.41 (n=468; 95% CI: 0.32, 0.52; p-value: <0.0001) and 0.48 (n=468; 95% CI: 0.38, 0.59; p-value: <0.0001), respectively. The median PFS was 5.6 months vs 1.7 months; the median OS was 12.1 months vs 6.7 months, in patients treated with sacituzumab govitecan and TPC, respectively.
The efficacy results in the overall population were consistent with the BMNeg population in the prespecified final analysis (11 March 2020 cut-off date) and are summarised in Table 3.
Table 3. Efficacy endpoints (overall population) - Pre-specified Final Analysis:
| Pre-specified Final Analysis (11 March 2020 cut-off date) | ||
|---|---|---|
| Sacituzumab govitecan n=267 | Treatment of physician's choice (TPC) n=262 | |
| Progression-free survival1 | ||
| Number of events (%) | 190 (71.2) | 171 (65.3) |
| Median PFS in months (95% CI) | 4.8 (4.1,5.8) | 1.7 (1.5, 2.5) |
| Hazard ratio (95% CI) | 0.43 (0.35, 0.54) | |
| p-value2 | <0.0001 | |
| Overall Survival | ||
| Number of deaths (%) | 179 (67.0) | 206 (78.6) |
| Median OS in months (95% CI) | 11.8 (10.5, 13.8) | 6.9 (5.9, 7.7) |
| Hazard ratio (95% CI) | 0.51 (0.41, 0.62) | |
| p-value2 | <0.0001 | |
| Overall response rate (ORR) | ||
| Number of responders (%) | 83 (31) | 11 (4) |
| Odds ratio (95% CI) | 10.99 (5.66, 21.36) | |
| p-value3 | <0.0001 | |
| Complete response, n (%) | 10 (4) | 2 (1) |
| Partial response, n (%) | 73 (27) | 9 (3) |
| Duration of response (DOR) | ||
| Median DOR in months (95% CI) | 6.3 (5.5, 9.0) | 3.6 (2.8, NE) |
1 PFS is defined as the time from the date of randomization to the date of the first radiological disease progression or death due to any cause, whichever comes first.
2 Stratified log-rank test adjusted for stratification factors: number of prior chemotherapies, presence of known brain metastases at study entry, and region.
3 Based on Cochran-Mantel-Haenszel test.
CI = Confidence Interval
In an updated efficacy analysis (final database lock 25 February 2021), results were consistent with the pre-specified final analysis. The median PFS by BICR was 4.8 months vs 1.7 months, in patients treated with sacituzumab govitecan and TPC, respectively (HR of 0.41; 95% CI: 0.33, 0.52). The median OS was 11.8 months vs 6.9 months, respectively (HR of 0.51; 95% CI: 0.42, 0.63). KaplanMeier curves for updated PFS by BICR and OS are presented in Figures 1 and 2.
Figure 1. Progression free survival (overall population; final database lock 25 February 2021) by BICR:
Figure 2. Overall survival (overall population; final database lock 25 February 2021):
Sub-group analysis
In subgroup analyses, improvements in PFS and OS in patients treated with sacituzumab govitecan compared to TPC were consistent across patient subgroups irrespective of age, race, BRCA status, prior number of systemic therapies overall (2 and >2, 2-3 and >3) and in the metastatic setting (1 and >1), prior therapy with anthracycline or PDL1, and liver metastases.
Brain metastases
An exploratory analysis of PFS and OS in patients with previously treated, stable brain metastases showed a stratified HR of 0.65 (n=61; 95% CI: 0.35, 1.22) and 0.87 (n=61; 95% CI: 0.47, 1.63), respectively. The median PFS was 2.8 months vs 1.6 months; the median OS was 6.8 months vs 7.5 months, in patients treated with sacituzumab govitecan and TPC, respectively.
Trop-2 expression
Additional subgroup analyses were conducted to evaluate the efficacy by tumour Trop-2 expression levels and the results were consistent across the different scoring methods used. In patients with low Trop-2 levels using membrane H-score by quartiles, benefit of sacituzumab govitecan over TPC was shown for both PFS (HR 0.64; 95% CI: 0.37, 1.11) and OS (HR of 0.71; 95% CI: 0.42, 1.21).
Unresectable or metastatic hormone receptor (HR)-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer (TROPiCS-02)
The efficacy of sacituzumab govitecan was evaluated in a multicentre, open-label, randomised study TROPiCS-02 (IMMU-132-09) conducted in 543 patients with unresectable locally advanced or metastatic HR-positive, HER2-negative (IHC 0, IHC 1+, or IHC 2+/ISH-) breast cancer whose disease has progressed after the following in any setting: a CDK 4/6 inhibitor, endocrine therapy, and a taxane; patients received at least two prior chemotherapies in the metastatic setting (one of which could be in the neoadjuvant or adjuvant setting if progression or recurrence occurred within 12 months of completion of the chemotherapy). Patients with bone-only disease, active chronic inflammatory bowel disease and known history of bowel obstruction, known history of unstable angina or myocardial infarction or congestive heart failure or active hepatitis B or C infection were excluded from the study.
Patients were randomised (1:1) to receive sacituzumab govitecan 10 mg/kg as an intravenous infusion on Days 1 and 8 of a 21-day cycle (n=272) or TPC (n=271). TPC was determined by the investigator before randomisation from one of the following single-agent regimens: eribulin (n=130), vinorelbine (n=63), gemcitabine (n=56), or capecitabine (n=22). Randomisation was stratified based on prior chemotherapy regimens for metastatic disease (2 vs. 3-4), visceral metastasis (yes vs. no), and endocrine therapy in the metastatic setting for at least 6 months (yes vs. no).
Patients were treated until disease progression or unacceptable toxicity. The primary efficacy outcome measure was PFS as determined by BICR per RECIST v1.1. Additional efficacy outcome measures were OS, ORR by BICR, and DOR by BICR.
The median age of the study population was 56 years (range: 27-86 years), and 26% of patients were 65 years or over. Almost all patients were female (99%). The majority of patients were White (67%); 4% were Black, 3% were Asian, and 26% were of unknown race. Patients received a median of 7 (range: 3 to 17) prior systemic regimens in any setting and 3 (range: 0 to 8) prior systemic chemotherapy regimens in the metastatic setting. Approximately 42% of patients had 2 prior chemotherapy regimens for metastatic disease compared to 58% of patients who had 3 to 4 prior chemotherapy regimens. Most patients received endocrine therapy in the metastatic setting for ≥6 months (86%). Patients had an ECOG performance status of 0 (44%) or 1 (56%). Ninety-five percent of patients had visceral metastases; 4.6% of patients had stable, pre-treated brain metastases.
Sacituzumab govitecan demonstrated a statistically significant improvement in PFS by BICR and OS versus TPC. The improvement in PFS by BICR and OS was generally consistent across pre-specified subgroups. Efficacy results are summarized in Table 4.
Table 4. Efficacy endpoints – Pre-specified Final Analysis:
| Sacituzumab govitecan n=272 | TPC n=271 | |
|---|---|---|
| Progression-Free Survival by BICR1 | ||
| Number of events (%) | 170 (62.5%) | 159 (58.7%) |
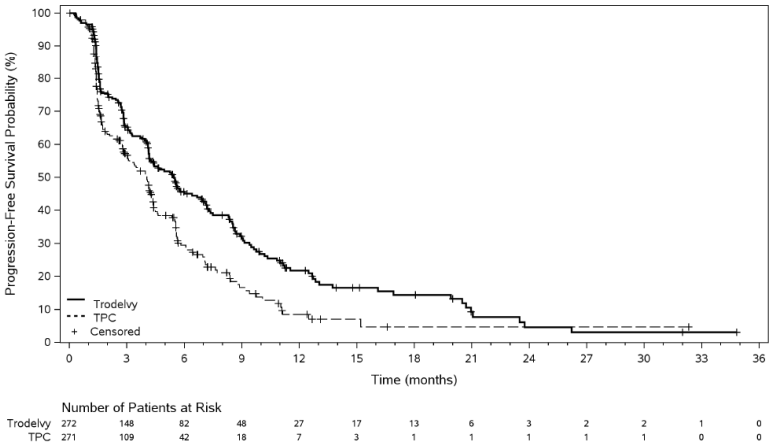
| Median PFS in months (95% CI) | 5.5 (4.2, 7.0) | 4.0 (3.1, 4.4) |
| Hazard ratio (95% CI) | 0.661 (0.529, 0.826) | |
| p-value2 | 0.0003 | |
| PFS rate at 12 months, % (95% CI) | 21.3 (15.2, 28.1) | 7.1 (2.8, 13.9) |
| Overall Survival3 | ||
| Number of events (%) | 191 (70.2%) | 199 (73.4%) |
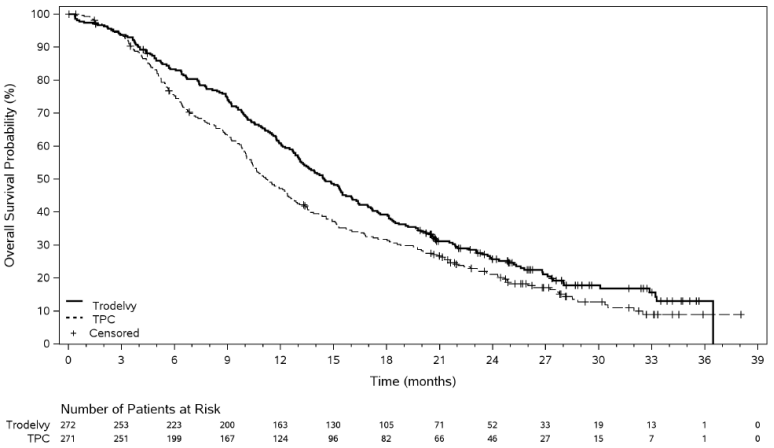
| Median OS in months (95% CI) | 14.4 (13.0, 15.7) | 11.2 (10.1, 12.7) |
| Hazard ratio (95% CI) | 0.789 (0.646, 0.964) | |
| p-value2 | 0.0200 | |
| Objective Response Rate by BICR3 | ||
| Number of responders (%) | 57 (21.0%) | 38 (14.0%) |
| Odds ratio (95% CI) | 1.625 (1.034, 2.555) | |
| p-value | 0.0348 | |
1 PFS is defined as the time from the date of randomisation to the date of the first radiological disease progression or death due to any cause, whichever comes first (data cut-off 3 January 2022).
2 Stratified log-rank test adjusted for stratification factors: prior chemotherapy regimens for metastatic disease (2 vs. 3-4), visceral metastasis (yes vs. no), and endocrine therapy in the metastatic setting for at least 6 months (yes vs. no).
3 Based on second interim OS analysis (data cut-off 1 July 2022).
BICR = Blinded Independent Central Review; CI = Confidence Interval
In an updated efficacy analysis with a median duration of follow-up of 12.8 months (data cut-off 1 December 2022), results were consistent with the pre-specified final analysis. The median PFS by BICR was 5.5 months vs 4.0 months, in patients treated with sacituzumab govitecan and TPC, respectively (HR of 0.65; 95% CI: 0.53, 0.81). The median OS was 14.5 months vs 11.2 months, respectively (HR of 0.79; 95% CI: 0.65, 0.95). Kaplan-Meier curves for updated PFS by BICR and OS are presented in Figures 3 and 4.
Figure 3. Progression free survival by BICR (data cut-off 1 December 2022):
Figure 4. Overall Survival (data cut-off 1 December 2022):
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with sacituzumab govitecan in all subsets of the paediatric population for the treatment of breast cancer (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The serum pharmacokinetics of sacituzumab govitecan and SN-38 were evaluated in the ASCENT study in a population of mTNBC patients who received sacituzumab govitecan as a single agent at a dose of 10 mg/kg body weight. The pharmacokinetic parameters of sacituzumab govitecan and free SN-38 are presented in Table 5.
Table 5. Summary of mean PK parameters (CV%) of sacituzumab govitecan and free SN-38:
| Sacituzumab govitecan | Free SN-38 | |
|---|---|---|
| Cmax [ng/mL] | 242 000 (22%) | 91 (65%) |
| AUC0-168 [ng*h/mL] | 5 560 000 (24%) | 2 730 (41%) |
Cmax: maximum serum concentration
AUC0-168: area under serum concentration curve through 168 hours
Distribution
Based on population pharmacokinetic analyses, the steady state volume of distribution of sacituzumab govitecan was 3.58 L.
Elimination
The median elimination half-life (t1/2) of sacituzumab govitecan and free SN-38 in patients with metastatic triple negative breast cancer was 23.4 and 17.6 hours, respectively. Based on population pharmacokinetic analyses, the clearance of sacituzumab govitecan is 0.128 L/h.
Metabolism
No metabolism studies with sacituzumab govitecan have been conducted. SN-38 (the small molecule moiety of sacituzumab govitecan) is metabolised via UGT1A1.
Special populations
Pharmacokinetic analyses in patients treated with sacituzumab govitecan (n=789) did not identify an effect of age, race, and mild or moderate renal impairment on the pharmacokinetics of sacituzumab govitecan.
Renal impairment
Renal elimination is known to contribute minimally to the excretion of SN-38, the small molecule moiety of sacituzumab govitecan. There are no data on the pharmacokinetics of sacituzumab govitecan in patients with severe renal impairment or end-stage renal disease (CrCl <15 mL/min).
Hepatic Impairment
The exposure of sacituzumab govitecan is similar in patients with mild hepatic impairment (bilirubin ≤ ULN and AST > ULN, or bilirubin >1.0 to ≤1.5 ULN and AST of any level; n=257) to patients with normal hepatic function (bilirubin and AST ≤ ULN; n=526). Sacituzumab govitecan and free SN-38 exposures are unknown in patients with moderate or severe hepatic impairment.
5.3. Preclinical safety data
SN-38 was clastogenic in an in vitro mammalian cell micronucleus test in Chinese hamster ovary cells and was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay. In a repeat-dose toxicity study in cynomolgus monkeys, intravenous administration of sacituzumab govitecan resulted in endometrial atrophy, uterine hemorrhage, increased follicular atresia of the ovary, and atrophy of vaginal epithelial cells at doses ≥60 mg/kg (1.9 times the human recommended dose of 10 mg/kg based on body weight allometric scaling).
Non-clinical data for the novel excipient MES reveal no special hazard for humans based on conventional repeated dose toxicity and genotoxicity studies.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.