TRUVELOG MIX 30 Suspension for injection Ref.[50036] Active ingredients: Insulin aspart
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: sanofi-aventis groupe, 54, rue La Boétie, F 75008 Paris, France
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, insulins and analogues for injection, intermediateor long-acting combined with fast-acting
ATC code: A10AD05
Truvelog Mix 30 is a biosimilar medicinal product. Detailed information is available on the website of the European Medicines Agency http://www.ema.europa.eu.
Mechanism of action and pharmacodynamic effects
Truvelog Mix 30 is a biphasic suspension of 30% soluble insulin aspart (rapid-acting human insulin analogue) and 70% protamine-crystallised insulin aspart (intermediate-acting human insulin analogue).
The blood glucose lowering effect of insulin aspart is due to the facilitated uptake of glucose following binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose output from the liver.
Truvelog Mix 30 is a biphasic insulin, which contains 30% soluble insulin aspart. This has a rapid onset of action, thus allowing it to be given closer to a meal (within zero to 10 minutes of the meal) when compared to soluble human insulin. The crystalline phase (70%) consists of protaminecrystallised insulin aspart, which has an activity profile similar to that of human NPH insulin.
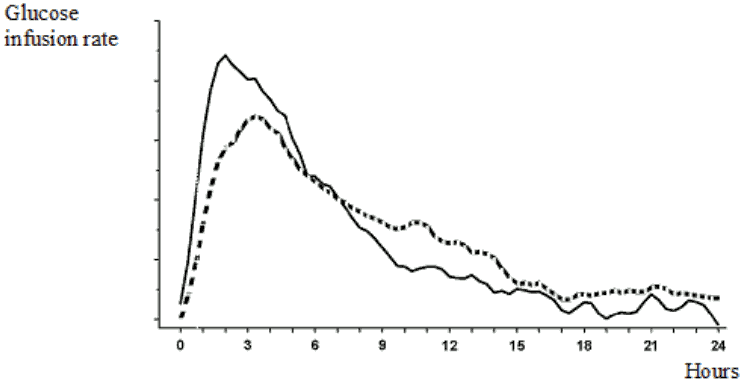
When Truvelog Mix 30 is injected subcutaneously, the onset of action will occur within 10 to 20 minutes of injection. The maximum effect is exerted between 1 and 4 hours after injection. The duration of action is up to 24 hours (Figure 1).
Figure 1. Activity profile of insulin aspart Mix 30 (−) and biphasic human insulin 30 (---) in healthy subjects:
Clinical efficacy and safety
In a 3-month trial in patients with type 1 and type 2 diabetes, insulin aspart Mix 30 showed equal control of glycosylated haemoglobin compared to treatment with biphasic human insulin 30. Insulin aspart is equipotent to human insulin on a molar basis. Compared to biphasic human insulin 30, administration of insulin aspart Mix 30 before breakfast and dinner resulted in lower postprandial blood glucose after both meals (breakfast and dinner).
A meta-analysis including nine trials in patients with type 1 and type 2 diabetes showed that fasting blood glucose was higher in patients treated with insulin aspart Mix 30, than in patients treated with biphasic human insulin 30.
In one trial, 341 patients with type 2 diabetes were randomised to treatment with insulin aspart Mix 30 either alone or in combination with metformin, or to metformin together with sulfonylurea. The primary efficacy variable – HbA1c after 16 weeks of treatment – did not differ between patients with insulin aspart Mix 30 combined with metformin and patients with metformin plus sulfonylurea. In this trial, 57% of the patients had baseline HbA1c above 9%; in these patients, treatment with insulin aspart Mix 30 in combination with metformin resulted in significantly lower HbA1c than metformin in combination with sulfonylurea.
In one trial, patients with type 2 diabetes, insufficiently controlled on oral hypoglycaemic agents alone, were randomised to treatment with twice daily insulin aspart Mix 30 (117 patients) or once daily insulin glargine (116 patients). After 28 weeks of treatment following the dosing guideline outlined in section 4.2, the mean reduction in HbA1c was 2.8% with insulin aspart Mix 30 (mean at baseline = 9.7%). With insulin aspart Mix 30, 66% and 42% of the patients reached HbA1c levels below 7% and 6.5%, respectively, and mean FPG was reduced by about 7 mmol/L (from 14 mmol/L at baseline to 7.1 mmol/L).
In patients with type 2 diabetes, a meta-analysis showed a reduced risk of overall nocturnal hypoglycaemic episodes and major hypoglycaemia with insulin aspart Mix 30 compared to biphasic human insulin 30. The risk of overall daytime hypoglycaemic episodes was increased in patients treated with insulin aspart Mix 30.
Paediatric population
A 16-week clinical trial comparing postprandial glycaemic control of meal-related insulin aspart Mix 30 with meal-related human insulin/biphasic human insulin 30 and bedtime NPH insulin was performed in 167 patients aged 10 to 18 years. Mean HbA1c remained similar to baseline throughout the trial in both treatment groups, and there was no difference in hypoglycaemia rate with insulin aspart Mix 30 or biphasic human insulin 30.
In a smaller (54 patients) and younger (age range 6 to 12 years) population, treated in a double-blind, cross-over trial (12 weeks on each treatment), the rate of hypoglycaemic episodes and the postprandial glucose increase were significantly lower with insulin aspart Mix 30 compared to biphasic human insulin 30. Final HbA1c was significantly lower in the biphasic human insulin 30 treated group compared with insulin aspart Mix 30.
5.2. Pharmacokinetic properties
Absorption, distribution and elimination
In insulin aspart, substitution of amino acid proline with aspartic acid at position B28 reduces the tendency to form hexamers as observed with soluble human insulin. The insulin aspart in the soluble phase of Truvelog Mix 30 comprises 30% of the total insulin; this is absorbed more rapidly from the subcutaneous layer than the soluble insulin component of biphasic human insulin. The remaining 70% is in crystalline form as protamine-crystallised insulin aspart; this has a prolonged absorption profile similar to human NPH insulin.
The maximum serum insulin concentration is, on average, 50% higher with insulin aspart Mix 30 than with biphasic human insulin 30. The time to maximum concentration is, on average, half of that for biphasic human insulin 30. In healthy volunteers, a mean maximum serum concentration of 140 ± 32 pmol/L was reached about 60 minutes after a subcutaneous dose of 0.20 unit/kg body weight. The mean half-life (t½) of insulin aspart Mix 30, reflecting the absorption rate of the protamine bound fraction, was about 8-9 hours. Serum insulin levels returned to baseline 15-18 hours after a subcutaneous dose. In type 2 diabetic patients, the maximum concentration was reached about 95 minutes after dosing, and concentrations well above zero for not less than 14 hours post-dosing were measured.
Special populations
The pharmacokinetics of insulin aspart Mix 30 have not been investigated in elderly patients or in patients with renal or hepatic impairment.
Paediatric population
The pharmacokinetics of insulin aspart Mix 30 have not been investigated in children or adolescents. However, the pharmacokinetic and pharmacodynamic properties of soluble insulin aspart have been investigated in children (6–12 years) and adolescents (13–17 years) with type 1 diabetes. Insulin aspart was rapidly absorbed in both age groups, with similar tmax as in adults. However, Cmax differed between the age groups, stressing the importance of the individual titration of insulin aspart.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and toxicity to reproduction and development.
In in vitro tests, including binding to insulin and IGF-1 receptor sites and effects on cell growth, insulin aspart behaved in a manner that closely resembled human insulin. Studies also demonstrate that the dissociation of binding to the insulin receptor of insulin aspart is equivalent to human insulin.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.