TUKYSA Film-coated tablet Ref.[49738] Active ingredients: Tucatinib
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Seagen B.V., Evert van de Beekstraat 1-104, 1118CL Schiphol, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinase inhibitors
ATC code: L01EH03
Mechanism of action
Tucatinib is a reversible, potent and selective tyrosine kinase inhibitor of HER2. In cellular signalling assays, tucatinib is >1000-fold more selective for HER2 compared to epidermal growth factor receptor. In vitro, tucatinib inhibits phosphorylation of HER2 and HER3, resulting in inhibition of downstream cell signalling and cell proliferation, and induces death in HER2 driven tumour cells. In vivo, tucatinib inhibits the growth of HER2 driven tumours and the combination of tucatinib and trastuzumab showed enhanced anti-tumour activity in vitro and in vivo compared to either medicinal product alone.
Pharmacodynamic effects
Cardiac electrophysiology
Multiple doses of tucatinib 300 mg twice a day did not have an effect on the QTc interval in a TQT study in healthy subjects.
Clinical efficacy and safety
The efficacy of tucatinib in combination with trastuzumab and capecitabine was evaluated in a randomised, double-blind, placebo-controlled, active comparator, global study (HER2CLIMB). Patients enrolled had locally advanced unresectable or metastatic HER2-positive breast cancer, with or without brain metastases, and had prior treatment with trastuzumab, pertuzumab, and trastuzumab emtansine (T-DM1) separately or in combination, in the neoadjuvant, adjuvant or metastatic setting. HER2 overexpression or amplification was confirmed by central laboratory analysis.
Patients with brain metastases, including those with untreated or progressing lesions, were eligible to enrol provided they were neurologically stable and did not require immediate brain radiation or surgery. Patients who required immediate local intervention could receive local therapy and be subsequently enrolled. The study included patients with untreated brain metastases and patients with treated brain metastases that were either stable or progressing since last brain radiation or surgery.
Patients were excluded from the study if they received systemic corticosteroids (≥2 mg total daily of dexamethasone or equivalent) for control of symptoms of CNS metastases <28 days prior to the first dose of study treatment. The study also excluded patients with leptomeningeal disease. Patients who had previously been treated with HER2 tyrosine kinase inhibitors were excluded with the exception of patients who received lapatinib for ≤21 days and was discontinued for reasons other than disease progression or severe toxicity. For patients with hormone receptor positive tumors, endocrine therapy was not permitted as concomitant therapy, with the exception of gonadotropin-releasing hormone agonists used for ovarian suppression in premenopausal women.
A total of 612 patients were randomised 2:1 to receive tucatinib in combination with trastuzumab and capecitabine (N=410) or placebo in combination with trastuzumab and capecitabine (N=202). Randomisation was stratified by the presence or history of brain metastases (yes vs. no), Eastern Cooperative Oncology Group (ECOG) performance status (0 vs. 1), and region (U.S., Canada, or rest of world).
Patient demographics were balanced between treatment arms. The median age was 54 years (range, 25 to 82); 116 (19%) patients were aged 65 years or older. 444 patients were white (73%) and 607 were female (99%). 314 patients (51%) had an ECOG performance status of 1 and 298 patients (49%) had an ECOG performance status of 0. Sixty percent had oestrogen and/or progesterone receptor-positive disease. Forty-eight percent of patients had a presence or history of brain metastases; of these, 23% had untreated brain metastases, 40% had treated but stable brain metastases, and 37% had treated but radiographically progressing brain metastases. Additionally, 49% of patients had lung metastases, 35% had liver metastases, and 14% had skin metastases. Patients had a median of 4 (range, 2 to 17) prior lines of systemic therapy and a median of 3 (range, 1 to 14) prior lines of systemic therapy in the metastatic setting. All patients received prior trastuzumab-based treatments and trastuzumab emtansine, while all but two patients had prior pertuzumab-based treatment.
Tucatinib or placebo, 300 mg orally twice per day, was administered until disease progression or unacceptable toxicity. Trastuzumab was administered intravenously as a loading dose of 8 mg/kg on Day 1 of Cycle 1, followed by a maintenance dose of 6 mg/kg on Day 1 of each subsequent 21-day cycle. An alternate dosing option for trastuzumab was a fixed dose of 600 mg administered subcutaneously on Day 1 of each 21-day cycle. Capecitabine, 1000 mg/m² orally twice per day, was administered on Days 1 through 14 of each 21-day cycle.
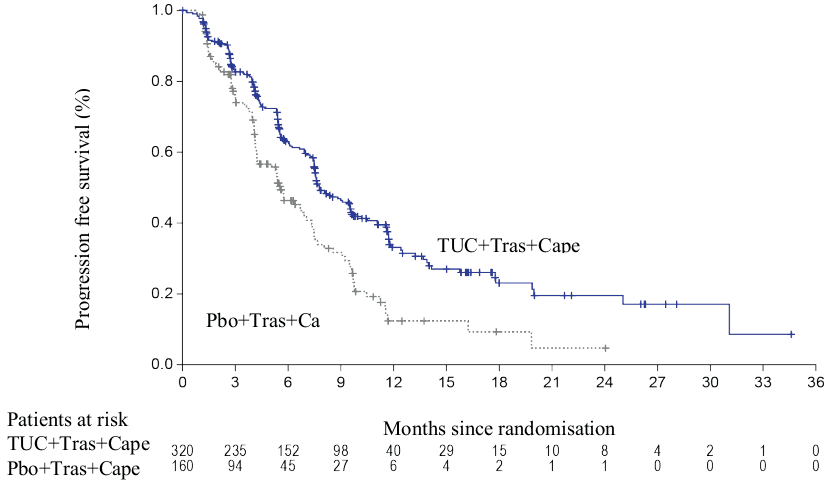
The primary endpoint was progression-free survival (PFS) by blinded independent central review (BICR) in the first 480 randomized patients. In this population, the median duration of exposure to tucatinib was 7.3 months (range <0.1, 35.1) for patients on the tucatinib + trastuzumab + capecitabine arm compared to 4.4 months (range <0.1, 24.0) of placebo for patients on the placebo + trastuzumab + capecitabine arm. Similar differences in exposure to trastuzumab and capecitabine were observed. Secondary endpoints were evaluated in all randomized patients (N=612) and included overall survival (OS), PFS among patients with a history or presence of brain metastases (PFSBrainMets) and confirmed objective response rate (ORR).
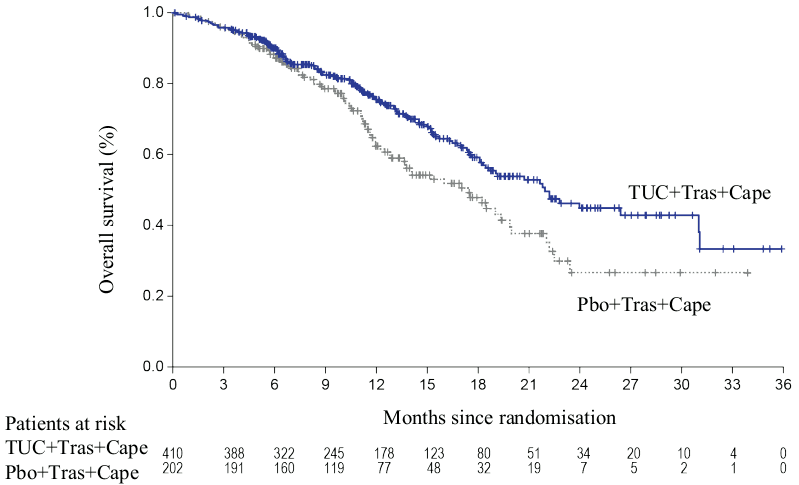
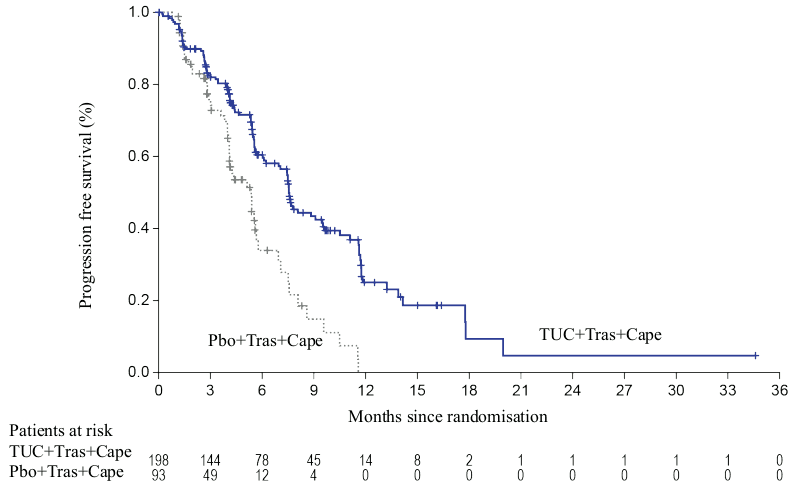
Efficacy results are summarized in Table 5 and Figures 1 to 3.
Primary and key secondary endpoint results were consistent across pre-specified subgroups: hormone receptor status, presence or history of brain metastases, ECOG status, and region. PFS as determined by the investigator was consistent with PFS as assessed by BICR.
Table 5. Efficacy results from the HER2CLIMB study:
| Tucatinib + Trastuzumab + Capecitabine | Placebo + Trastuzumab + Capecitabine | |
|---|---|---|
| PFS1 | N=320 | N=160 |
| Number of events (%) | 178 (56) | 97 (61) |
| Hazard ratio (95% CI)2 | 0.54 (0.42, 0.71) | |
| P-value3 | <0.00001 | |
| Median (months) (95% CI) | 7.8 (7.5, 9.6) | 5.6 (4.2, 7.1) |
| OS | N=410 | N=202 |
| Number of deaths, n (%) | 130 (32) | 85 (42) |
| Hazard ratio (95% CI)2 | 0.66 (0.50, 0.87) | |
| P-value3 | 0.00480 | |
| Median OS, months (95% CI) | 21.9 (18.3, 31.0) | 17.4 (13.6, 19.9) |
| PFSBrainMets4 | N=198 | N=93 |
| Number of events (%) | 106 (53.5) | 51 (54.8) |
| Hazard ratio (95% CI)2 | 0.48 (0.34, 0.69) | |
| P-value3 | <0.00001 | |
| Median (months) (95% CI) | 7.6 (6.2, 9.5) | 5.4 (4.1, 5.7) |
| Confirmed ORR for Patients with Measurable Disease | N=340 | N=171 |
| ORR (95% CI)5 | 40.6 (35.3, 46.0) | 22.8 (16.7, 29.8) |
| P-value6 | 0.00008 | |
| CR (%) | 3 (0.9) | 2 (1.2) |
| PR (%) | 135 (39.7) | 37 (21.6) |
| DOR | ||
| Median DOR in months (95% CI)7 | 8.3 (6.2, 9.7) | 6.3 (5.8, 8.9) |
BICR = blinded independent central review; CI = confidence interval; PFS = progression-free survival; OS=overall survival; ORR = objective response rate; CR = complete response; PR = partial response; DOR = duration of response.
1 Primary PFS analysis conducted in first 480 randomized patients. PFS based on Kaplan-Meier analyses.
2 Hazard ratio and 95% confidence intervals are based on stratified Cox proportional hazards regression model controlling for stratification factors (presence or history of brain metastases, Eastern Cooperative Oncology Group (ECOG) status, and region of world).
3 Two-sided p-value based on re-randomization procedure controlling for stratification factors.
4 Analysis includes patients with history or presence of parenchymal brain metastases at baseline, including target and non-target lesions. Does not include patients with dural lesions only.
5 Two-sided 95% exact confidence interval, computed using the Clopper-Pearson method.
6 Cochran-Mantel-Haenszel test controlling for stratification factors (presence or history of brain metastases, Eastern Cooperative Oncology Group (ECOG) status, and region of world).
7 Calculated using the complementary log-log transformation method.
Figure 1. Kaplan-Meier curves of progression-free survival (per BICR):
Figure 2. Kaplan-Meier curves of overall survival:
Figure 3. Kaplan-Meier curves of progression-free survival (per BICR) in patients with brain metastases:
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with TUKYSA in all subsets of the paediatric population in malignant breast neoplasms (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Plasma tucatinib exposure (AUCinf and Cmax) demonstrated dose proportional increases at oral doses from 50 to 300 mg (0.17 to 1 time the recommended dose). Tucatinib exhibited 1.7-fold accumulation for AUC and 1.5-fold accumulation for Cmax following administration of 300 mg tucatinib twice daily for 14 days. Time to steady-state was approximately 4 days.
Absorption
Following a single oral dose of 300 mg tucatinib, the median time to peak plasma concentration was approximately 2.0 hours (range 1.0 to 4.0 hours).
Effects of food
Following administration of a single dose of tucatinib in 11 subjects after a high-fat meal (approximately 58% fat, 26% carbohydrate, and 16% protein), the mean AUCinf increased by 1.5-fold, the Tmax shifted from 1.5 hours to 4.0 hours, and Cmax was unaltered. The effect of food on the pharmacokinetics of tucatinib was not clinically meaningful, thus tucatinib may be administered without regard to food.
Distribution
The apparent volume of distribution of tucatinib was approximately 1670 L in healthy subjects after a single dose of 300 mg. The plasma protein binding was 97.1% at clinically relevant concentrations.
Biotransformation
Tucatinib is metabolized primarily by CYP2C8 and to a lesser extent via CYP3A and aldehyde oxidase.
In Vitro drug interaction studies
Tucatinib is a substrate of CYP2C8 and CYP3A.
Tucatinib is a reversible inhibitor of CYP2C8 and CYP3A and a time-dependent inhibitor of CYP3A, at clinically relevant concentrations.
Tucatinib has low potential to inhibit CYP1A2, CYP2B6, CYP2C9, CYP2C19, CYP2D6, and UGT1A1 at clinically relevant concentrations.
Tucatinib is a substrate of P-gp and BCRP. Tucatinib is not a substrate of OAT1, OAT3, OCT1, OCT2, OATP1B1, OATP1B3, MATE1, MATE2-K, and BSEP.
Tucatinib inhibits MATE1/MATE2-K-mediated transport of metformin and OCT2/MATE1-mediated transport of creatinine. The observed serum creatinine increase in clinical studies with tucatinib is due to inhibition of tubular secretion of creatinine via OCT2 and MATE1.
Elimination
Following a single oral dose of 300 mg, tucatinib is cleared from plasma with a geometric mean halflife of approximately 8.5 hours and apparent clearance of 148 L/h in healthy subjects.
Excretion
Tucatinib is predominantly eliminated by the hepatobiliary route and is not appreciably renally eliminated. Following a single oral dose of 300 mg 14C-tucatinib, approximately 85.8% of the total radiolabelled dose was recovered in faeces (15.9% of the administered dose as unchanged tucatinib) and 4.1% in urine with an overall total recovery of 89.9% within 312 hours post-dose. In plasma, approximately 75.6% of the plasma radioactivity was unchanged, 19% was attributed to identified metabolites, and approximately 5% was unassigned.
Special populations
Based on population pharmacokinetic analysis according to demographic characteristics, age (<65 years (N=211); ≥65 years (N=27)), albumin (25.0 to 52.0 g/L), creatinine clearance (CLcr 60 to 89 mL/min (N=89); CLcr 30 to 59 mL/min (N=5)), body weight (40.7 to 138.0 kg), and race (White (N=168), Black (N=53), or Asian (N=10)) did not have a clinically meaningful effect on tucatinib exposure. There are no data for subjects with severely impaired renal function.
Renal impairment
The pharmacokinetics of tucatinib have not been evaluated in a dedicated renal impairment study.
Hepatic impairment
Mild (Child–Pugh A) and moderate (Child-Pugh B) hepatic impairment had no clinically relevant effect on tucatinib exposure. Tucatinib AUCinf was increased by 1.6-fold in subjects with severe (Child-Pugh C) hepatic impairment compared to subjects with normal hepatic function. There are no data for breast cancer patients with severely impaired hepatic function.
5.3. Preclinical safety data
Carcinogenicity studies have not been conducted with tucatinib.
Tucatinib was not clastogenic or mutagenic in the standard battery of genotoxicity assays.
In repeat-dose toxicity studies in rats, decreased corpora lutea/corpus luteum cyst, increased interstitial cells of the ovary, atrophy of the uterus, and mucification of the vagina were observed at doses of ≥6 mg/kg/day administered twice daily, equivalent to 0.09 times the human exposure based on AUC0-12 at the recommended dose. No histological effects were observed on male or female reproductive tracts in cynomolgus monkeys or on male reproductive tracts in rats at doses resulting in exposures up to 8 times (in monkey) or 13 times (in rat) the human exposure at the recommended dose based on AUC0-12.
Embryo-foetal development studies were conducted in rabbits and rats. In pregnant rabbits, increased resorptions, decreased percentages of live foetuses, and skeletal, visceral, and external malformations were observed in foetuses at ≥90 mg/kg/day; at this dose, maternal exposure is approximately equivalent to the human exposure at the recommended dose based on AUC. In pregnant rats, decreased maternal body weight and body weight gain were observed at doses of ≥90 mg/kg/day. Foetal effects of decreased weights and delayed ossification were observed at ≥120 mg/kg/day; at this dose, maternal exposure is approximately 6-fold higher than human exposure at the recommended dose based on AUC.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.