TYPHIM VI Solution for injection Ref.[11099] Active ingredients: Typhoid purified polysaccharide antigen
Source: FDA, National Drug Code (US) Revision Year: 2021
3. Indications and Usage
Typhim Vi vaccine is indicated for active immunization for the prevention of typhoid fever caused by S typhi and is approved for use in persons two years of age or older.
Immunization with Typhim Vi vaccine should occur at least two weeks prior to expected exposure to S typhi.
Typhim Vi vaccine is not indicated for routine immunization of individuals in the United States (US). (14)
Selective immunization against typhoid fever is recommended under the following circumstances: 1) travelers to areas where a recognized risk of exposure to typhoid exists, particularly ones who will have prolonged exposure to potentially contaminated food and water, 2) persons with intimate exposure (ie, continued household contact) to a documented typhoid carrier, and 3) workers in microbiology laboratories who frequently work with S typhi. (14)
Typhoid vaccination is not required for international travel, but is recommended for travelers to such areas as Africa, Asia, and Central and South America where there is a recognized risk of exposure to S typhi. Current CDC advisories should be consulted with regard to specific locales. Vaccination is particularly recommended for travelers who will have prolonged exposure to potentially contaminated food and water. However, even travelers who have been vaccinated should use caution in selecting food and water. (15)
There is no evidence to support the use of typhoid vaccine to control common source outbreaks, disease following natural disaster or in persons attending rural summer camps. (14)
An optimal reimmunization schedule has not been established. Reimmunization every two years under conditions of repeated or continued exposure to the S typhi organism is recommended at this time.
For recommended primary immunization and reimmunization see DOSAGE AND ADMINISTRATION section.
Typhim Vi vaccine should not be used to treat a patient with typhoid fever or a chronic typhoid carrier.
10. Dosage and Administration
For intramuscular use only.
Dosage
The immunizing dose for adults and children is a single injection of 0.5 mL.
A reimmunizing dose is 0.5 mL. Reimmunization consisting of a single dose for US travelers every two years under conditions of repeated or continued exposure to the S typhi organism is recommended at this time. (14)
Preparation for Administration
The syringe or vial and its packaging should be inspected prior to use for evidence of leakage, premature activation of the plunger, or a faulty tip seal. If any of these conditions exists, do NOT administer the vaccine.
Syringe
Picture A: Luer-Lok syringe
| Step 1: Holding the syringe cap in one hand (avoid holding the syringe plunger or barrel), unscrew the tip cap by twisting it counterclockwise. | 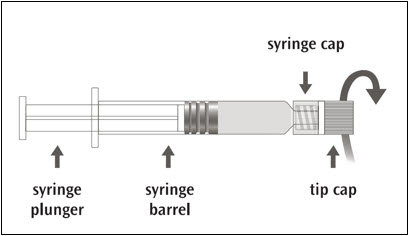 |
| Step 2: To attach the needle to the syringe, gently twist the needle clockwise into the syringe until slight resistance is felt. |  |
The syringe is intended for single use only, must not be reused, and must be disposed of properly and promptly following its use.
Vial
Tear off upper seal of vial cap. Cleanse top of rubber stopper of the vial with a suitable antiseptic.
Use a separate sterile syringe and needle or a sterile disposable unit for each individual patient to prevent the transmission of infectious agents from person to person. Needles should not be recapped and should be properly disposed.
Administration
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If any of this conditions exists, do NOT administer the vaccine.
In adults, the intramuscular injection is typically given in the deltoid. In children, the intramuscular injection is given either in the deltoid or the anterolateral thigh.
Do NOT inject this vaccine into the gluteal area or areas where there may be a nerve trunk.
Do NOT inject intravenously.
12. Storage and Handling
Store at 2° to 8°C (35° to 46°F). DO NOT FREEZE. Discard if the vaccine has been frozen.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.