ULTRAVATE Lotion Ref.[11105] Active ingredients: Ulobetasol
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
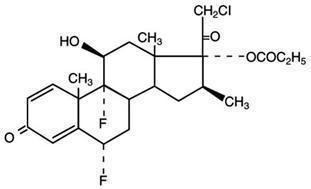
ULTRAVATE (halobetasol propionate) lotion, 0.05% for topical use contains a corticosteroid, halobetasol propionate. The chemical name of halobetasol propionate is 21-chloro-6α, 9-difluoro-11β, 17-dihydroxy-16β-methylpregna-1, 4-diene-3-20-dione, 17-propionate. Halobetasol propionate is a white to off-white crystalline powder with a molecular weight of 484.96 and a molecular formula of C25H31ClF2O5. It is practically insoluble in water and freely soluble in dichloromethane and in acetone.
It has the following structural formula:
Each gram of ULTRAVATE lotion contains 0.5 mg of halobetasol propionate in a white to off-white lotion base consisting of diisopropyl adipate, octyldodecanol, ceteth-20, poloxamer 407, cetyl alcohol, stearyl alcohol, propylparaben, butylparaben, propylene glycol, glycerin, carbomer homopolymer, sodium hydroxide, and water.
| Dosage Forms and Strengths |
|---|
|
ULTRAVATE (halobetasol propionate) lotion, 0.05% is a white to off-white lotion. Each gram of ULTRAVATE lotion contains 0.5 mg of halobetasol propionate. |
| How Supplied |
|---|
|
ULTRAVATE lotion, 0.05% is white to off-white lotion. It is supplied in an oval tapered white high-density polyethylene bottle with a white polypropylene disc cap. Each bottle contains 60 mL (59 g) of ULTRAVATE lotion. NDC 10631-122-04 60 mL (59 g) bottle Manufactured by: Ferndale Laboratories, Inc., Ferndale, MI 48220 Distributed by: Sun Pharmaceutical Industries, Inc., Cranbury, NJ 08512 |
Drugs
| Drug | Countries | |
|---|---|---|
| ULTRAVATE | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.