UPTRAVI Film-coated tablet Ref.[8623] Active ingredients: Selexipag
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: Antithrombotic agents, platelet aggregation inhibitors excluding heparin
ATC code: B01AC27
Mechanism of action
Selexipag is a selective IP receptor agonist distinct from prostacyclin and its analogues. Selexipag is hydrolysed by carboxylesterases to yield its active metabolite, which is approximately 37-fold more potent than selexipag. Selexipag and the active metabolite are high-affinity IP receptor agonists with a high selectivity for the IP receptor versus other prostanoid receptors (EP1–EP4, DP, FP, and TP). Selectivity against EP1, EP3, FP, and TP is important because these are well-described contractile receptors in the gastro-intestinal tract and blood vessels. Selectivity against EP2, EP4, and DP1 is important because these receptors mediate immune depressive effects.
Stimulation of the IP receptor by selexipag and the active metabolite leads to vasodilatory as well as anti-proliferative and anti-fibrotic effects. Selexipag prevents cardiac and pulmonary remodelling in a rat model of PAH and causes proportional decreases in pulmonary and peripheral pressures, indicating that peripheral vasodilation reflects pulmonary pharmacodynamic efficacy. Selexipag does not cause IP receptor desensitisation in vitro nor tachyphylaxis in a rat model.
Pharmacodynamic effects
Cardiac electrophysiology
In a thorough QT study in healthy subjects, repeated doses of 800 and 1,600 micrograms of selexipag twice daily did not show an effect on cardiac repolarisation (QTc interval) or conduction (PR and QRS intervals) and had a mild accelerating effect on heart rate (the placebo-corrected, baseline-adjusted increase in heart rate reached 6–7 bpm at 1.5 to 3 h after dosing with 800 micrograms selexipag and 9-10 bpm at the same timepoints after 1,600 micrograms selexipag).
Coagulation factors
In Phase 1 and 2 studies a slight decrease in plasma levels of von Willebrand factor (vWF) was observed with selexipag; the vWF values remained above the lower limit of the normal range.
Pulmonary haemodynamics
A Phase 2 double-blind, placebo-controlled clinical study assessed haemodynamic variables after 17 weeks of treatment in patients with PAH WHO FC II–III and concomitantly receiving ERAs and/or PDE-5 inhibitors. Patients titrating selexipag to an individually tolerated dose (200 micrograms twice daily increments up to 800 micrograms twice daily; N=33) achieved a statistically significant mean reduction in pulmonary vascular resistance of 30.3% (95% confidence interval [CI]: −44.7%, −12.2%; p=0.0045) and an increase in cardiac index (mean treatment effect) of 0.48 L/min/m² (95% CI: 0.13, 0.83) compared to placebo (N=10).
Clinical efficacy and safety
Efficacy in patients with PAH
The effect of selexipag on progression of PAH was demonstrated in a multi-centre, long-term (maximum duration of exposure approximately 4.2 years), double-blind, placebo-controlled, parallel-group, event-driven Phase 3 study in 1,156 patients with symptomatic (WHO FC I–IV) PAH. Patients were randomised to either placebo (N=582) or selexipag (N=574) twice daily. The dose was increased at weekly intervals by increments of 200 micrograms given twice daily to determine the individualised maintenance dose (200–1,600 micrograms twice daily).
The primary study endpoint was the time to first occurrence of a morbidity or mortality event up to end of treatment, defined as a composite of death (all causes); or hospitalisation for PAH; or progression of PAH resulting in need for lung transplantation or balloon atrial septostomy; or initiation of parenteral prostanoid therapy or chronic oxygen therapy; or other disease-progression events (patients in WHO FC II or III at baseline) confirmed by a decrease in 6-minute walk distance (6MWD) from baseline (≥15%) and worsening of WHO FC or (patients in WHO FC III or IV at baseline) confirmed by a decrease in 6MWD from baseline (≥15%) and need for additional PAH-specific therapy.
All events were confirmed by an independent adjudication committee, blinded to treatment allocation.
The mean age was 48.1 years (range 18–80 years of age), with the majority of subjects being Caucasian (65.0%) and female (79.8%). 17.9% of patients were ≥65 and 1.1% ≥75 years of age. Approximately 1%, 46%, 53%, and 1% of patients were in WHO FC I, II, III and IV, respectively, at baseline.
Idiopathic or heritable PAH was the most common aetiology in the study population (58%) followed by PAH due to connective tissue disorders (29%), PAH associated with simple corrected congenital heart disease (10%), and PAH associated with other aetiologies (drugs and toxins [2%] and HIV [1%]).
At baseline, the majority of enrolled patients (80%) were being treated with a stable dose of a specific therapy for PAH, either an ERA (15%) or a PDE-5 inhibitor (32%) or both an ERA and a PDE-5 inhibitor (33%).
The overall median double-blind treatment duration was 63.7 weeks for the placebo group and 70.7 weeks for the selexipag group. 23% of patients on selexipag achieved maintenance doses in the range of 200–400 micrograms, 31% achieved doses in the range of 600–1,000 micrograms, and 43% achieved doses in the range of 1,200–1,600 micrograms.
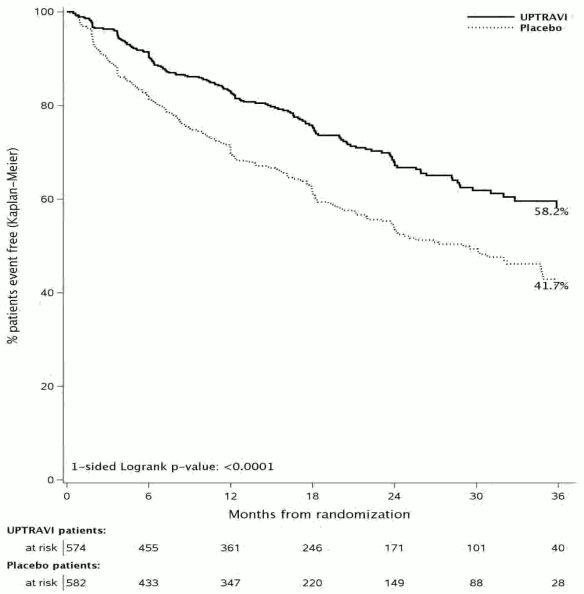
Treatment with selexipag 200–1,600 micrograms twice daily resulted in a 40% reduction (hazard ratio [HR] 0.60; 99% CI: 0.46, 0.78; one-sided log-rank p value<0.0001) of the occurrence of morbidity or mortality events up to 7 days after last dose compared to placebo (Figure 1). The beneficial effect of selexipag was primarily attributable to a reduction in hospitalisation for PAH and a reduction in other disease-progression events (Table 1).
Figure 1. Kaplan-Meier estimates of the first morbidity-mortality event:
Table 1. Summary of outcome events:
| Endpoints & statistics | Patients with an event | Treatment comparison: selexipag vs placebo | ||||
|---|---|---|---|---|---|---|
| Placebo (Ν=582) | Selexipa g (Ν=574) | Absolute risk reduction | Relative risk reduction (99% CI) | HR (99% CI) | p-value | |
| Morbidity-mortality eventa | 58,3% | 41,8% | 16,5% | 40% (22%, 54%) | 0,60 (0,46, 0,78) | <0,0001 |
| Hospitalisation due to PAHb n (%) | 109 (18,7%) | 78 (13,6%) | 5,1% | 33% (2%, 54%) | 0,67 (0,46, 0,98) | 0,04 |
| Disease progressionb n (%) | 100 (17,2%) | 38 (6,6%) | 10,6% | 64% (41%, 78%) | 0,36 (0,22, 0,59) | <0,0001 |
| i.v./s.c. Prostanoid initiation or oxygen therapybc n (%) | 15 (2,6%) | 11 (1,9%) | 0,7% | 32% (−90%, 76%) | 0,68 (0,24, 1,90) | 0,53 |
| Death up to EOT + 7 daysd n (%) | 37 (6,4%) | 46 (8,0%) | −1,7% | −17% (−107%, 34%) | 1,17 (0,66, 2,07) | 0,77 |
| Death up to study closured n (%) | 105 (18,0%) | 100 (17,4%) | 0,6% | 3% (−39%, 32%) | 0,97 (0,68, 1,39) | 0,42 |
CI = confidence interval; EOT = end of treatment; HR = hazard ratio; i.v. = intravenous; PAH = pulmonary arterial hypertension; s.c. = subcutaneous.
a % of patients with an event at 36 months = 100 × (1 – Kaplan-Meier estimate); hazard ratio estimated using Cox’s proportional hazard model; unstratified one-sided log-rank p-value
b % of patients with an event as part of the primary endpoint up to EOT + 7 days; hazard ratio estimated using Aalen Johansen method; 2-sided p-value using Gray’s test
c Includes ‘Need for lung transplantation or atrial septostomy’ (1 patient on selexipag and 2 on placebo)
d % of patients with an event up to EOT + 7 days or up to study closure; hazard ratio estimated using Cox’s proportional hazard model; unstratified one-sided log-rank p-value
The numerical increase in deaths up to end of treatment + 7 days but not up to study closure was further investigated by mathematical modelling, showing that the imbalance in deaths is consistent with the assumption of a neutral effect on PAH mortality and reduction of non-fatal events.
The observed effect of selexipag versus placebo on the primary endpoint was consistent across individualised maintenance dose as shown by the hazard ratio for the three pre-defined categories (0.60 for 200–400 micrograms twice daily, 0.53 for 600–1,000 micrograms twice daily, and 0.64 for 1,200–1,600 micrograms twice daily), which was consistent with the overall treatment effect (0.60).
The efficacy of selexipag on the primary endpoint was consistent across subgroups of age, sex, race, aetiology, geographical region, WHO FC, and as monotherapy or in combination with an ERA or a PDE-5 inhibitor or triple combination with both an ERA and a PDE-5 inhibitor.
Time to PAH-related death or hospitalisation for PAH was assessed as a secondary endpoint. The risk of an event for this endpoint was reduced by 30% in patients receiving selexipag compared to placebo (HR 0.70, 99% CI: 0.50, 0.98; one-sided log-rank p=0.0031). The percentages of patients with an event at Month 36 were 28.9% and 41.3% in the selexipag and placebo groups, respectively, with an absolute risk reduction of 12.4%.
The number of patients who experienced, as a first event, death due to PAH or hospitalisation for PAH up to end of treatment was 102 (17.8%) in the selexipag group and 137 (23.5%) in the placebo group. Death due to PAH as a component of the endpoint was observed in 16 (2.8%) patients on selexipag and 14 (2.4%) on placebo. Hospitalisation for PAH was observed in 86 (15.0%) patients on selexipag and 123 (21.1%) patients on placebo. Selexipag reduced the risk of hospitalisation for PAH as a first outcome event compared to placebo (HR 0.67, 99% CI: 0.46, 0.98; one-sided log-rank p=0.04).
The total number of deaths of all causes up to study closure was 100 (17.4%) for the selexipag group and 105 (18.0%) for the placebo group (HR 0.97, 99% CI: 0.68, 1.39). The number of deaths due to PAH up to study closure was 70 (12.2%) for the selexipag group and 83 (14.3%) for the placebo group.
Symptomatic endpoints
Exercise capacity was evaluated as a secondary endpoint. Median 6MWD at baseline was 376 m (range: 90–482 m) and 369 m (range: 50–515 m) in selexipag patients and placebo patients, respectively. Treatment with selexipag resulted in a placebo-corrected median effect on 6MWD measured at trough (i.e. approximately 12 h post-dose) of 12 m at Week 26 (99% CI: 1, 24 m; one-sided p value=0.0027). In patients without concurrent PAH-specific therapy, the placebo-corrected treatment effect measured at trough was 34 m (99% CI: 10, 63 m).
Quality of life was assessed in a subset of patients in the GRIPHON study using the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) questionnaire. There was no significant treatment effect from baseline to Week 26.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Uptravi in one or more subsets of the paediatric population for treatment of pulmonary hypertension, (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
The pharmacokinetics of selexipag and its active metabolite have been studied primarily in healthy subjects. The pharmacokinetics of selexipag and the active metabolite, both after single- and multiple-dose administration, were dose-proportional up to a single dose of 800 micrograms and multiple doses of up to 1,800 micrograms twice daily. After multiple-dose administration, steady state conditions of selexipag and the active metabolite were reached within 3 days. No accumulation in plasma, either of parent compound or active metabolite, occurred after multiple-dose administration.
In healthy subjects, inter-subject variability in exposure (area under the curve over a dosing interval) at steady state was 43% and 39% for selexipag and the active metabolite, respectively. Intra-subject variability in exposure was 24% and 19% for selexipag and the active metabolite, respectively.
Exposure to selexipag and the active metabolite at steady state in PAH patients and healthy subjects was similar. The pharmacokinetics of selexipag and the active metabolite in PAH patients were not influenced by the severity of the disease and did not change with time.
Absorption
Selexipag is rapidly absorbed and is hydrolysed by carboxylesterases to its active metabolite.
Maximum observed plasma concentrations of selexipag and its active metabolite after oral administration are reached within 1–3 h and 3–4 h, respectively.
The absolute bioavailability of selexipag in humans is approximately 49%. This is most probably due to a first-pass effect of selexipag, as plasma concentrations of the active metabolite are similar after the same oral and intravenous dose administration.
In the presence of food, the exposure to selexipag after a single dose of 400 micrograms was increased by 10% in Caucasian subjects and decreased by 15% in Japanese subjects, whereas exposure to the active metabolite was decreased by 27% (Caucasian subjects) and 12% (Japanese subjects). More subjects reported adverse events after administration in the fasted than in the fed state.
Distribution
Selexipag and its active metabolite are highly bound to plasma proteins (approximately 99% in total and to the same extent to albumin and alpha1-acid glycoprotein). The volume of distribution of selexipag at steady state is 11.7 L.
Biotransformation
Selexipag is hydrolysed to its active metabolite in the liver and in the intestine by carboxylesterases. Oxidative metabolism catalysed mainly by CYP2C8 and to a smaller extent by CYP3A4 leads to the formation of hydroxylated and dealkylated products. UGT1A3 and UGT2B7 are involved in the glucuronidation of the active metabolite. Except for the active metabolite, none of the circulating metabolites in human plasma exceed 3% of the total drug-related material. Both in healthy subjects and PAH patients, after oral administration, exposure at steady state to the active metabolite is approximately 3- to 4-fold higher than to the parent compound.
Elimination
Elimination of selexipag is predominantly via metabolism with a mean terminal half-life of 0.8–2.5 h. The active metabolite has a half-life of 6.2–13.5 h. The total body clearance of selexipag is 17.9 L/h. Excretion in healthy subjects was complete 5 days after administration and occurred primarily via faeces (accounting for 93% of the administered dose) compared to 12% in urine.
Special populations
No clinically relevant effects of sex, race, age, or body weight on the pharmacokinetics of selexipag and its active metabolite have been observed in healthy subjects or PAH patients.
Renal impairment
A 1.4- to 1.7-fold increase in exposure (maximum plasma concentration and area under the plasma concentration-time curve) to selexipag and its active metabolite was observed in subjects with severe renal impairment (eGFR <30 mL/min/1.73 m²).
Hepatic impairment
In subjects with mild (Child-Pugh class A) or moderate (Child-Pugh class B) hepatic impairment, exposure to selexipag was 2- and 4-fold higher, respectively, when compared to healthy subjects. Exposure to the active metabolite remained almost unchanged in subjects with mild hepatic impairment and was doubled in subjects with moderate hepatic impairment. Only two subjects with severe (Child-Pugh class C) hepatic impairment were dosed with selexipag. Exposure to selexipag and its active metabolite in these two subjects was similar to that in subjects with moderate (Child-Pugh class B) hepatic impairment.
Based on modelling and simulation data from a study in subjects with hepatic impairment, the exposure to selexipag at steady state in subjects with moderate hepatic impairment (Child-Pugh class B) after a once-daily regimen is predicted to be approximately 2-fold higher than that in healthy subjects during a twice-daily regimen. The exposure to the active metabolite at steady state in these patients during a once-daily regimen is predicted to be similar to that in healthy subjects during a twice-daily regimen. Subjects with severe hepatic impairment (Child-Pugh class C) showed similar predicted exposure at steady state as subjects with moderate hepatic impairment during a once-daily regimen.
Preclinical safety data
In the repeated-dose toxicity studies in rodents, strong blood pressure decrease as a result of exaggerated pharmacology induced transient clinical signs and reduced food consumption and body-weight gain. In adult and juvenile dogs, intestine and bone/bone marrow were identified as the main target organs after treatment with selexipag. A delay in the closure of the femoral and/or tibial epiphyseal growth plate was observed in juvenile dogs. A no-observed-adverse-effect level was not established. In juvenile dogs, intussusception due to prostacyclin-related effects on intestinal motility was observed sporadically. Safety margins adapted for IP receptor potency for the active metabolite were 2-fold (based on total exposure) in relation to human therapeutic exposure. The finding did not occur in mouse or rat toxicity studies. Because of the species-specific sensitivity of dogs to develop intussusception, this finding is considered not relevant for adult humans.
Increased bone ossification and related changes in the bone marrow in dog studies are considered to be due to the activation of EP4 receptors in dogs. As human EP4 receptors are not activated by selexipag or its active metabolite, this effect is species-specific and, therefore, not relevant to humans. Selexipag and the active metabolite are not genotoxic on the basis of the overall evidence of conducted genotoxicity studies.
In the 2-year carcinogenicity studies, selexipag caused an increased incidence of thyroid adenomas in mice and Leydig cell adenomas in rats. The mechanisms are rodent-specific. Tortuosity of retinal arterioles was noted after 2 years of treatment only in rats. Mechanistically, the effect is considered to be induced by life-long vasodilation and subsequent changes in ocular haemodynamics. Additional histopathological findings of selexipag were observed only at exposures sufficiently in excess of the maximum human exposure, indicating little relevance to humans.
In a fertility study performed in rats, a prolongation of oestrus cycles resulting in increases in days until copulation was observed at exposures 173-fold above therapeutic exposures (based on total exposures), the no-observed-effect level being 30-fold above therapeutic exposures. Otherwise, fertility parameters were not affected.
Selexipag was not teratogenic in rats and rabbits (exposure margins above therapeutic exposure of 13-fold for selexipag and 43-fold for the active metabolite, based on total exposure). Safety margins for potential IP receptor-related effects on reproduction were 20 for fertility and 5 and 1 (based on free exposure) for embryo-foetal development in rats and rabbits, respectively, when adapted for differences in receptor potency. In the rat pre-/post-natal development study, selexipag induced no effects on maternal and pup reproductive function.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.