URAPIDIL Solution for injection Ref.[7677] Active ingredients: Urapidil
Therapeutic indications
Hypertensive emergencies (e.g. a critical rise in blood pressure), severe and very severe forms of hypertensive disease, hypertension resistant to treatment.
Controlled lowering of blood pressure in hypertensive patients during and/or after surgery.
Posology and method of administration
For hypertensive emergencies, severe and very severe forms of hypertension, and treatment resistant hypertension
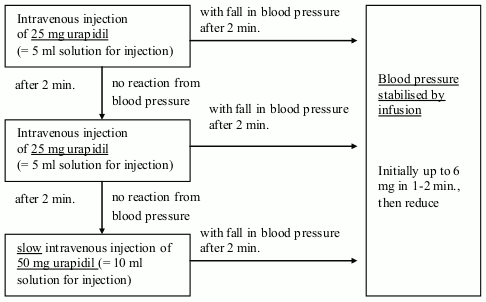
Intravenous injection: 10-50 mg urapidil is slowly administered by intravenous injection - while constantly monitoring the blood pressure. A fall in blood pressure can be expected within 5 min. of administering the injection.
The injection of 10-50 mg urapidil can be repeated depending how the blood pressure reacts.
Intravenous infusion or syringe pump are used to maintain the level of blood pressure achieved by the injection.
For instructions on dilution of the medicinal product before administration, see section 6.6.
The maximum quantity compatible is 4 mg urapidil per ml of solution for infusion.
Speed of administration: The infusion rate is determined from the individual blood pressure situation. Initial recommended maximum infusion rate: 2 mg/min.
Maintenance dose: On average 9 mg/h, referring to 250 mg urapidil added to 500 ml solution for infusion corresponding to 1 mg = 44 drops = 2.2 ml.
Controlled lowering of blood pressure when blood pressure is raised during and/or after surgery
Intravenous infusion or syringe pump is used to maintain the level of blood pressure achieved by the injection.
Dosage regimen
Note
Urapidil Stragen i.v. is administered intravenously as an injection or infusion to supine patients. The dose may be administered as one or several injections or as slow intravenous infusion. Injections can be combined with subsequent slow infusion.
Elderly
In elderly patients antihypertensive agents must be administered with appropriate care and at the beginning in small doses, as in these patients sensitivity to these kinds of preparations is often modified.
Patients with kidney and/or liver function disorders
In patients with kidney and/or liver function disorders it may be necessary to reduce the dose of urapidil.
Paediatric population
Safety and efficacy of intravenous urapidil in children aged 0-18 years have not been established.
No recommendation on a posology can be made.
Duration of treatment
A period of treatment of 7 days has been found to be safe from a toxicological point of view; with parenteral antihypertensive drugs this period should also not, in general, be exceeded. Renewed parenteral treatment is possible if the blood pressure rises again.
It is possible to overlap acute parenteral therapy with changing to continuous treatment with oral agents that lower blood pressure.
Overdose
Symptoms
Symptoms of overdose are dizziness, orthostatic hypotension and collapse as well as fatigue and decreased reactivity.
Treatment of overdose
An excessive fall in blood pressure can be corrected by raising the legs and performing volume replacement. If these measures are not sufficient, vasoconstricting preparations can be slowly injected intravenously while monitoring the blood pressure. In very rare cases the administration of catecholamines (e.g. adrenaline, 0.5-1.0 mg diluted to 10 ml with isotonic sodium chloride solution) is necessary.
Shelf life
3 years.
After first opening/dilution: Chemical and physical in use stability has been demonstrated for 50 hours at 15-25°C.
From the microbiological point of view, the product should be used immediately.
If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution/dilution has been taken place in controlled and validated conditions.
Special precautions for storage
Do not store above 30°C.
For storage conditions of the diluted medicinal product, see section 6.3.
Nature and contents of container
Ampoules of clear glass (type I Ph. Eur.)
Pack size: 5 ampoules.
Special precautions for disposal and other handling
The 100 mg ampoule can only be used for stabilisation of blood pressure by infusion.
For the initial treatment ampoules containing 25 mg and 50 mg urapidil are available. These dosage strengths can also be used for intravenous infusion after dilution.
The dilution is made under aseptic conditions.
The solution should be expected visually for particulate matter and discoloration prior to administration. Only clear and colourless solution should be used.
Preparation of diluted solution:
Intravenous infusion: Add 250 mg urapidil (2 ampoules of 100 mg urapidil + 1 ampoule of 50 mg urapidil) to 500 ml of one of the compatible solvents.
Syringe pump: 100 mg urapidil is drawn up into a syringe pump and diluted to a volume of 50 ml with one of the compatible solvents.
Compatible solvents for dilution:
- Sodium chloride 9 mg/ml (0.9%) solution for infusion
- Glucose 50 mg/ml (5%)
- Glucose 100 mg/ml (10%)
For single use only.
Any unused solution and the "bags/sachets" should be adequately disposed of, in accordance with local requirements".
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.