UROXATRAL Extended-release tablet Ref.[49799] Active ingredients: Alfuzosin
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Each UROXATRAL extended-release tablet contains 10 mg alfuzosin hydrochloride as the active ingredient. Alfuzosin hydrochloride is a white to off-white crystalline powder that melts at approximately 240°C. It is freely soluble in water, sparingly soluble in alcohol, and practically insoluble in dichloromethane.
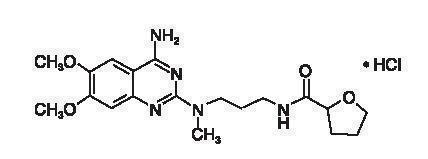
Alfuzosin hydrochloride is (R,S)N[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl) methylamino] propyl] tetrahydro-2-furancarboxamide hydrochloride. The empirical formula of alfuzosin hydrochloride is C19H27N5O4•HCl. The molecular weight of alfuzosin hydrochloride is 425.9.
Its structural formula is:
The tablet also contains the following inactive ingredients: colloidal silicon dioxide (NF), ethylcellulose (NF), hydrogenated castor oil (NF), hydroxypropyl methylcellulose (USP), magnesium stearate (NF), mannitol (USP), microcrystalline cellulose (NF), povidone (USP), and yellow ferric oxide (NF).
| Dosage Forms and Strengths |
|---|
|
UROXATRAL (alfuzosin HCl) extended-release tablet 10 mg is available as a round, three-layer tablet: one white layer between two yellow layers, debossed with X10. |
| How Supplied |
|---|
|
UROXATRAL is supplied as follows: Package NDC Number Bottles of 100 with child-resistant closure, NDC 59212-200-10 UROXATRAL (alfuzosin HCl) extended-release tablet 10 mg is available as a round, three-layer tablet: one white layer between two yellow layers, debossed with X10. |
Drugs
| Drug | Countries | |
|---|---|---|
| UROXATRAL | Germany, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.