URSO 250 / FORTE Film-coated tablet Ref.[11137] Active ingredients: Ursodeoxycholic acid
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
URSO 250 (ursodiol, 250 mg) is available as a film-coated tablet for oral administration. URSO Forte (ursodiol, 500 mg) is available as a scored film-coated tablet for oral administration.
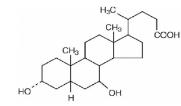
Ursodiol (ursodeoxycholic acid, UDCA) is a naturally occurring bile acid found in small quantities in normal human bile and in larger quantities in the biles of certain species of bears. It is a bitter-tasting white powder consisting of crystalline particles freely soluble in ethanol and glacial acetic acid, slightly soluble in chloroform, sparingly soluble in ether, and practically insoluble in water. The chemical name of ursodiol is 3α,7ß-dihydroxy-5ß-cholan-24-oic (C24H40O4). Ursodiol has a molecular weight of 392.56.
Its structure is shown below.
Inactive ingredients: microcrystalline cellulose, povidone, sodium starch glycolate, magnesium stearate, ethylcellulose, dibutyl sebacate, carnauba wax, hydroxypropyl methylcellulose, PEG 3350, PEG 8000, cetyl alcohol, sodium lauryl sulfate and hydrogen peroxide.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
16.1 URSO 250Each URSO 250 elliptical, biconvex, film-coated tablet, white, engraved with "URS785", contains 250 mg of ursodiol. Available in bottles of 100 tablets (NDC 58914-785-10). 16.2 URSO ForteEach URSO Forte elliptical, biconvex, scored, film-coated tablet, white, engraved with "URS790", contains 500 mg of ursodiol. Available in bottles of 100 tablets (NDC 58914-790-10). Distributed by: Allergan USA, Inc., Madison, NJ 07940, www.allergan.com |
Drugs
| Drug | Countries | |
|---|---|---|
| URSO | Canada, Japan, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.