UVADEX Solution for injection Ref.[11072] Active ingredients: Methoxsalen
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
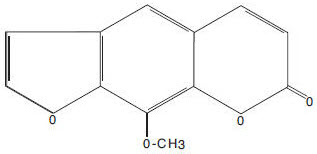
Methoxsalen is a naturally occurring photoactive substance found in the seeds of the Ammi majus (Umbelliferae) plant. It belongs to a group of compounds known as psoralens or furocoumarins. The chemical name of methoxsalen is 9-methoxy-7H-furo[3,2-g][1]-benzopyran-7-one; it has the following structure:
Each mL of UVADEX (methoxsalen, 8-methoxypsoralen) Sterile Solution contains methoxsalen 20 mcg, propylene glycol 50 mg, sodium chloride 8 mg, sodium acetate 1.75 mg, ethanol 40.550 mg, glacial acetic acid 1.260 mg, and Water for Injection q.s. to 1.0 mL. Glacial acetic acid and sodium hydroxide are used to adjust the pH of the solution if necessary. UVADEX is a clear, colorless to pale yellow liquid.
UVADEX is used in combination with the THERAKOS CELLEX Photopheresis System to extracorporeally treat leukocyte enriched buffy coat.
| How Supplied |
|---|
|
UVADEX (methoxsalen) Sterile Solution 20 mcg/mL in 10 mL amber glass vials (NDC 64067-216-01), and cartons of 12 vials (NDC 64067-216-01). One vial of 10 mL contains 200 micrograms methoxsalen. Manufactured by Patheon Manufacturing Services LLC, Greenville, NC 27834 |
Drugs
| Drug | Countries | |
|---|---|---|
| UVADEX | Austria, Australia, Canada, France, Poland, Turkey, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.