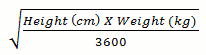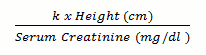VALGANCICLOVIR Film-coated tablets Ref.[8518] Active ingredients: Valganciclovir
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Zentiva Pharma UK Limited, 12 New Fetter Lane, London, EC4A 1JP, United Kingdom Trading as: Zentiva, 12 New Fetter Lane, London EC4A 1JP, UK
Therapeutic indications
Valganciclovir is indicated for the induction and maintenance treatment of cytomegalovirus (CMV) retinitis in adult patients with acquired immunodeficiency syndrome (AIDS).
Valganciclovir is indicated for the prevention of CMV disease in CMV-negative adults and children (aged from birth to 18 years) who have received a solid organ transplant from a CMV-positive donor.
Posology and method of administration
Posology
Caution – Strict adherence to dosage recommendations is essential to avoid overdose (see sections 4.4 and 4.9).
Valganciclovir is rapidly and extensively metabolised to ganciclovir after oral dosing. Oral valganciclovir 900 mg b.i.d. is therapeutically equivalent to intravenous ganciclovir 5 mg/kg b.i.d.
Treatment of cytomegalovirus (CMV) retinitis
Adult patients
Induction treatment of CMV retinitis:
For patients with active CMV retinitis, the recommended dose is 900 mg valganciclovir (two Valganciclovir 450 mg tablets) twice a day for 21 days and, whenever possible, taken with food. Prolonged induction treatment may increase the risk of bone marrow toxicity (see section 4.4).
Maintenance treatment of CMV retinitis:
Following induction treatment, or in patients with inactive CMV retinitis, the recommended dose is 900 mg valganciclovir (two Valganciclovir 450 mg tablets) once daily and, whenever possible, taken with food. Patients whose retinitis worsens may repeat induction treatment; however, consideration should be given to the possibility of viral drug resistance.
The duration of maintenance treatment should be determined on an individual basis.
Paediatric population
The safety and efficacy of valganciclovir in the treatment of CMV retinitis have not been established in adequate and well-controlled clinical studies in paediatric patients.
Prevention of CMV disease in solid organ transplantation
Adult patients
For kidney transplant patients, the recommended dose is 900 mg (two Valganciclovir 450 mg tablets) once daily, starting within 10 days post-transplantation and continuing until 100 days post-transplantation. Prophylaxis may be continued until 200 days post-transplantation (see sections 4.4, 4.8 and 5.1).
For patients who have received a solid organ transplant other than kidney, the recommended dose is 900 mg (two Valganciclovir 450 mg tablets) once daily, starting within 10 days post- transplantation and continuing until 100 days post-transplantation.
Whenever possible, the tablets should be taken with food.
Paediatric population
In paediatric solid organ transplant patients, aged from birth, who are at risk of developing CMV disease, the recommended once daily dose of valganciclovir is based on body surface area (BSA) and creatinine clearance (Clcr) derived from Schwartz formula (ClcrS), and is calculated using the equation below:
Paediatric Dose (mg) = 7 x BSA x ClcrS (see Mosteller BSA formula and Schwartz Creatinine Clearance formula below).
If the calculated Schwartz creatinine clearance exceeds 150 mL/min/1.73m², then a maximum value of 150 mL/min/1.73m² should be used in the equation:
Mosteller BSA (m²) =
Schwartz Creatinine Clearance (ml/min/1,73m²) =
Where k = 0.45* for patients aged <2 years, 0.55 for boys aged 2 to <13 years and girls aged 2 to 16 years, and 0.7 for boys aged 13 to 16 years. Refer to adult dosing for patients older than 16 years of age.
The k values provided are based on the Jaffe method of measuring serum creatinine and may require correction when enzymatic methods are used.
* For appropriate sub-populations a lowering of k value may also be necessary (e.g. in paediatric patients with low birth weight).
For paediatric kidney transplant patients, the recommended once daily mg dose (7 x BSA x ClcrS) should start within 10 days post-transplantation and continue until 200 days post-transplantation.
For paediatric patients who have received a solid organ transplant other than kidney, the recommended once daily mg dose (7x BSA x ClcrS) should start within 10 days post transplantation and continue until 100 days post-transplantation.
All calculated doses should be rounded to the nearest 25 mg increment for the actual deliverable dose. If the calculated dose exceeds 900 mg, a maximum dose of 900 mg should be administered. The oral solution is the preferred formulation since it provides the ability to administer a dose calculated according to the formula above; however, valganciclovir film-coated tablets may be used if the calculated doses are within 10% of available tablet doses, and the patient is able to swallow tablets. For example, if the calculated dose is between 405 mg and 495 mg, one 450 mg tablet may be taken.
It is recommended to monitor serum creatinine levels regularly and consider changes in height and body weight and adapt the dose as appropriate during the prophylaxis period.
Special dosage instructions
Paediatric population
Dosing of paediatric SOT patients is individualised based on a patient's renal function, together with body surface area.
Elderly patients
Safety and efficacy have not been established in this patient population. No studies have been conducted in adults older than 65 years of ages. Since renal clearance decreases with age, valganciclovir should be administered to elderly patients with special consideration of their renal status (see table below). (See section 5.2)
Patients with renal impairment
Serum creatinine levels or estimated creatinine clearance should be monitored carefully. Dosage adjustment is required according to creatinine clearance, as shown in the table below (see sections 4.4 and 5.2).
An estimated creatinine clearance (ml/min) can be related to serum creatinine by the following formulae:
For males = (140 - age [years]) X (body weight [kg]) / (72) X (0.011 X serum creatinine [μmol/l])
For females = 0.85 X male value
| Creatinine clearance (CLcr) (ml/min) | Induction dose of valganciclovir | Maintenance/Prevention dose of valganciclovir |
|---|---|---|
| ≥60 | 900 mg (2 tablets) twice daily | 900 mg (2 tablets) once daily |
| 40–59 | 450 mg (1 tablet) twice daily | 450 mg (1 tablet) once daily |
| 25–39 | 450 mg (1 tablet) once daily | 450 mg (1 tablet) every 2 days |
| 10–24 | 450 mg (1 tablet) every 2 days | 450 mg (1 tablet) twice weekly |
| <10 | Not recommended | Not recommended |
Patients undergoing haemodialysis
For patients on haemodialysis (Clcr < 10 ml/min) a dose recommendation cannot be given. Thus Valganciclovir film-coated tablets should not be used in these patients (see sections 4.4 and 5.2).
Patients with hepatic impairment
Safety and efficacy of valganciclovir tablets have not been established in patients with hepatic impairment (see section 5.2).
Patients with severe leukopenia, neutropenia, anaemia, thrombocytopenia and pancytopenia
See section 4.4 before initiation of therapy. If there is a significant deterioration of blood cell counts during therapy with valganciclovir, treatment with haematopoietic growth factors and/or dose interruption should be considered (see section 4.4).
Method of administration
Valganciclovir is administered orally, and whenever possible, should be taken with food (see section 5.2).
Valganciclovir is also available as an oral suspension for use in patients who are unable to swallow tablets (please refer to the Summary of Product Characteristics for oral suspension containing valganciclovir).
Precautions to be taken before handling or administering the medicinal product
The tablets should not be broken or crushed. Since valganciclovir is considered a potential teratogen and carcinogen in humans, caution should be observed in handling broken tablets (see section 4.4). Avoid direct contact of broken or crushed tablets with skin or mucous membranes. If such contact occurs, wash thoroughly with soap and water, rinse eyes thoroughly with sterile water, or plain water if sterile water is unavailable.
Overdose
Overdose experience with valganciclovir and intravenous ganciclovir
It is expected that an overdose of valganciclovir could possibly result in increased renal toxicity (see sections 4.2 and 4.4).
Reports of overdoses with intravenous ganciclovir, some with fatal outcomes, have been received from clinical trials and during post-marketing experience. In some of these cases no adverse events were reported. The majority of patients experienced one or more of the following adverse events:
- Haematological toxicity: myelosuppression including pancytopenia, bone marrow failure, leukopenia, neutropenia, granulocytopenia.
- Hepatotoxicity: hepatitis, liver function disorder.
- Renal toxicity: worsening of haematuria in a patient with pre-existing renal impairment, acute kidney injury, elevated creatinine.
- Gastrointestinal toxicity: abdominal pain, diarrhoea, vomiting.
- Neurotoxicity: generalised tremor, seizure.
Haemodialysis and hydration may be of benefit in reducing blood plasma levels in patients who receive an overdose of valganciclovir (see section 5.2).
Shelf life
Shelf life: 24 months.
Special precautions for storage
This medicinal product does not require any special storage conditions.
Nature and contents of container
High density polyethylene (HDPE) bottles and child resistant polypropylene (PP) screw cap with induction heat sealing (with aluminum foil). The screw cap has a tamper evident.
Pack size: One bottle containing 60 film coated tablets.
Special precautions for disposal and other handling
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.