VALIUM Tablet Ref.[11138] Active ingredients: Diazepam
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
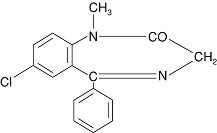
Valium (diazepam) is a benzodiazepine derivative. The chemical name of diazepam is 7-chloro-1,3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one. It is a colorless to light yellow crystalline compound, insoluble in water. The empirical formula is C16H13ClN2O and the molecular weight is 284.75.
The structural formula is as follows:
Valium is available for oral administration as tablets containing 2 mg, 5 mg or 10 mg diazepam. In addition to the active ingredient diazepam, each tablet contains the following inactive ingredients: anhydrous lactose, corn starch, pregelatinized starch and calcium stearate with the following dyes: 5-mg tablets contain FD&C Yellow No. 6 and D&C Yellow No. 10; 10-mg tablets contain FD&C Blue No. 1. Valium 2-mg tablets contain no dye.
| How Supplied |
|---|
|
For oral administration, Valium is supplied as round, flat-faced scored tablets with V-shaped perforation and beveled edges. Valium is available as follows: 2 mg, white - bottles of 100 (NDC 0140-0004-01); 5 mg, yellow - bottles of 100 (NDC 0140-0005-01) and 500 (NDC 0140-0005-14); 10 mg, blue - bottles of 100 (NDC 0140-0006-01) and 500 (NDC 0140-0006-14). Engraved on tablets: 2 mg—2 VALIUM (front) 5 mg—5 VALIUM (front) 10 mg—10 VALIUM (front) Distributed by: Roche Laboratories Inc. on behalf of Roche Products Inc., 150 Clove Road, Suite 8, Little Falls, NJ 07424 |
Drugs
| Drug | Countries | |
|---|---|---|
| VALIUM | Australia, Brazil, Canada, Spain, France, Malta, Mexico, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.