VARGATEF Soft capsule Ref.[8899] Active ingredients: Nintedanib
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Boehringer Ingelheim International GmbH, Binger Strasse 173, 55216 Ingelheim am Rhein, Germany
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, protein kinase inhibitors
ATC code: L01EX09
Mechanism of action
Nintedanib is a triple angiokinase inhibitor blocking vascular endothelial growth factor receptors (VEGFR 1-3), platelet-derived growth factor receptors (PDGFR α and ß) and fibroblast growth factor receptors (FGFR 1-3) kinase activity. Nintedanib binds competitively to the adenosine triphosphate (ATP) binding pocket of these receptors and blocks the intracellular signalling which is crucial for the proliferation and survival of endothelial as well as perivascular cells (pericytes and vascular smooth muscle cells). In addition Fms-like tyrosine-protein kinase (Flt)-3, lymphocyte-specific tyrosine-protein kinase (Lck) and proto-oncogene tyrosine-protein kinase Src (Src) are inhibited.
Pharmacodynamic effects
Tumour angiogenesis is an essential feature contributing to tumour growth, progression and metastasis formation and is predominantly triggered by the release of pro-angiogenic factors secreted by the tumour cell (i.e. VEGF and bFGF) to attract host endothelial as well as perivascular cells to facilitate oxygen and nutrient supply through the host vascular system. In preclinical disease models nintedanib, as single agent, effectively interfered with the formation and maintenance of the tumour vascular system resulting in tumour growth inhibition and tumour stasis. In particular, treatment of tumour xenografts with nintedanib led to a rapid reduction in tumour micro vessel density, pericytes vessel coverage and tumour perfusion.
Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) measurements showed an anti-angiogenic effect of nintedanib in humans. It was not clearly dose dependent, but most responses were seen at doses of ≥200 mg. Logistic regression revealed a statistically significant association of the anti-angiogenic effect to nintedanib exposure. DCE-MRI effects were seen 24-48 h after the first intake of the medicinal product and were preserved or even increased after continuous treatment over several weeks. No correlation of the DCE-MRI response and subsequent clinically significant reduction in target lesion size was found, but DCE-MRI response was associated with disease stabilization.
Clinical efficacy and safety
Efficacy in the pivotal phase 3 trial LUME-Lung 1
The efficacy and safety of Vargatef was investigated in 1 314 adult patients with locally advanced, metastatic or recurrent NSCLC after one prior line of chemotherapy. ‘Locally recurrent’ was defined as local re-occurrence of the tumour without metastases at trial entry. The trial included 658 patients (50.1%) with adenocarcinoma, 555 patients (42.2%) with squamous cell carcinoma, and 101 patients (7.7%) with other tumour histologies.
Patients were randomized (1:1) to receive nintedanib 200 mg orally twice daily in combination with 75 mg/m² of intravenous docetaxel every 21 days (n=655) or placebo orally twice daily in combination with 75 mg/m² of docetaxel every 21 days (n=659). Randomization was stratified according to Eastern Cooperative Oncology Group (ECOG) status (0 versus 1), bevacizumab pretreatment (yes versus no), brain metastasis (yes versus no) and tumour histology (squamous versus non-squamous tumour histology).
Patient characteristics were balanced between treatment arms within the overall population and within subgroups according to histology. In the overall population, 72.7% of the patients were male. The majority of patients were non-Asian (81.6%), the median age was 60.0 years, the baseline ECOG performance status was 0 (28.6%) or 1 (71.3%); one patient had a baseline ECOG performance status of 2. Five point eight percent (5.8%) of the patients had stable brain metastasis at trial entry and 3.8% had prior bevacizumab treatment.
The disease stage was determined at the time of diagnosis using Union Internationale Contre le Cancer (UICC)/American Joint Committee on Cancer (AJCC) Edition 6 or Edition 7. In the overall population, 16.0% of the patients had disease stage < IIIB/IV, 22.4%, had disease stage IIIB and 61.6% had disease stage IV. 9.2% of the patients entered the trial with locally recurrent disease stage as had been evaluated at baseline. For patients with tumour of adenocarcinoma histology, 15.8% had disease stage < IIIB/IV, 15.2%, had disease stage IIIB and 69.0% had disease stage IV. 5.8% of the adenocarcinoma patients entered the trial with locally recurrent disease stage as had been evaluated at baseline.
The primary endpoint was progression-free survival (PFS) as assessed by an independent review committee (IRC) based on the intent-to-treat (ITT) population and tested by histology. Overall survival (OS) was the key secondary endpoint. Other efficacy outcomes included objective response, disease control, change in tumour size and health-related quality of life.
The addition of nintedanib to docetaxel led to a statistically significant reduction in the risk of progression or death by 21% for the overall population (hazard ratio (HR) 0.79; 95% confidence interval (CI): 0.68-0.92; p=0.0019) as determined by the Independent Review Committee. This result was confirmed in the follow-up PFS analysis (HR 0.85, 95% CI: 0.75-0.96; p=0.0070) which included all events collected at the time of the final OS analysis. Overall survival analysis in the overall population did not reach statistical significance (HR 0.94; 95% CI: 0.83-1.05). Of note, pre-planned analyses according to histology showed statistically significant difference in OS between treatment arms in the adenocarcinoma population only (Table 4).
As shown in Table 4, the addition of nintedanib to docetaxel led to a statistically significant reduction in the risk of progression or death by 23% for the adenocarcinoma population (HR 0.77; 95% CI: 0.62-0.96). In line with these observations, related trial endpoints such as disease control and change in tumour size showed significant improvements.
Table 4. Efficacy results for trial LUME-Lung 1 for patients with adenocarcinoma tumour histology:
| Vargatef + Docetaxel | Placebo + Docetaxel | |
|---|---|---|
| Progression free survival (PFS)* – primary analysis | ||
| Patients, n | 277 | 285 |
| Number of Deaths or Progressions, n (%) | 152 (54.9) | 180 (63.2) |
| Median PFS [months] | 4.0 | 2.8 |
| HR (95% CI) | 0.77 (0.62; 0.96) | |
| Stratified Log-Rank Test p-value** | 0.0193 | |
| Progression free survival (PFS)*** – follow-up analysis | ||
| Patients, n | 322 | 336 |
| Number of Deaths or Progressions, n (%) | 255 (79.2) | 267 (79.5) |
| Median PFS [months] | 4.2 | 2.8 |
| HR (95% CI) | 0.84 (0.71; 1.00) | |
| Stratified Log-Rank Test p-value** | 0.0485 | |
| Disease control [%] | 60.2 | 44.0 |
| Odds ratio (95% CI)+ | 1.93 (1.42; 2.64) | |
| p-value+ | <0.0001 | |
| Objective response [%] | 4.7 | 3.6 |
| Odds ratio (95% CI)+ | 1.32 (0.61; 2.93) | |
| p-value+ | 0.4770 | |
| Tumour shrinkage [%]° | -7.76 | -0.97 |
| p-value° | 0.0002 | |
| Overall Survival (OS)*** | ||
| Patients, n | 322 | 336 |
| Number of Deaths, n (%) | 259 (80.4) | 276 (82.1) |
| Median OS [months] | 12.6 | 10.3 |
| HR (95% CI) | 0.83 (0.70; 0.99) | |
| Stratified Log-Rank Test p-value* | 0.0359 | |
HR: hazard ratio; CI: confidence interval
* Primary PFS analysis performed when 713th PFS events had been observed based on IRC-assessment in the overall ITT population (332 events in adenocarcinoma patients).
** Stratified by baseline ECOG PS (0 versus 1), brain metastases at baseline (yes versus no) and prior treatment with bevacizumab (yes versus no).
*** OS analysis and follow-up PFS-analysis performed when 1 121 death cases had been observed in the overall ITT population (535 events in adenocarcinoma patients).
+ Odds ratio and p-value were obtained from a logistic regression model adjusted for baseline ECOG Performance Score (0 versus 1).
° Adjusted mean of best-% change from baseline and p-value generated from an ANOVA model adjusting for baseline ECOG PS (0 versus 1), brain metastases at baseline (yes versus no) and prior treatment with bevacizumab (yes versus no).
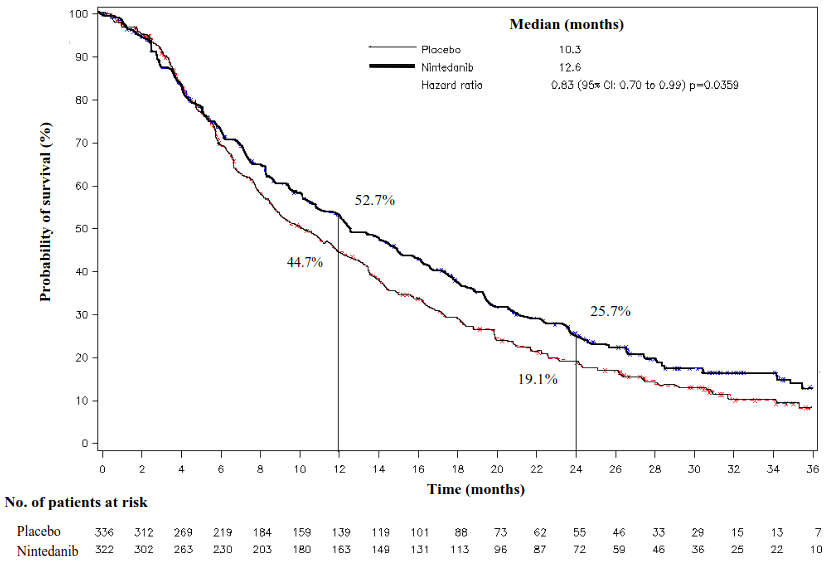
A statistically significant improvement in OS favouring treatment with nintedanib plus docetaxel was demonstrated in patients with adenocarcinoma with a 17% reduction in the risk of death (HR 0.83, p=0.0359) and a median OS improvement of 2.3 months (10.3 versus 12.6 months, Figure 1).
Figure 1. Kaplan-Meier curve for overall survival for patients with adenocarcinoma tumour histology by treatment group in trial LUME-Lung 1:
A pre-specified evaluation was performed in the population of adenocarcinoma patients considered to have entered the trial with a particularly poor treatment prognosis, namely, patients who progressed during or shortly after first-line therapy prior to trial entry. This population included those adenocarcinoma patients identified at baseline as having progressed and entered the trial less than 9 months since start of their first-line therapy. Treatment of these patients with nintedanib in combination with docetaxel reduced the risk of death by 25%, compared with placebo plus docetaxel (HR 0.75; 95% CI: 0.60-0.92; p=0.0073). Median OS improved by 3 months (nintedanib: 10.9 months; placebo: 7.9 months). In a post-hoc analysis in adenocarcinoma patients having progressed and entered the trial ≥9 months since start of their first-line therapy the difference did not reach statistical significance (HR for OS: 0.89, 95% CI 0.66-1.19).
The proportion of adenocarcinoma patients with stage < IIIB/IV at diagnosis was small and balanced across treatment arms (placebo: 54 patients (16.1%); nintedanib: 50 patients, (15.5%)). The HR for these patients for PFS and OS was 1.24 (95% CI: 0.68, 2.28) and 1.09 (95% CI: 0.70, 1.70), respectively. However, the sample size was small, there was no significant interaction and the CI was wide and included the HR for OS of the overall adenocarcinoma population.
Quality of life
Treatment with nintedanib did not significantly change the time to deterioration of the pre-specified symptoms cough, dyspnoea and pain, but resulted in a significant deterioration in the diarrhoea symptom scale. Nevertheless, the overall treatment benefit of nintedanib was observed without adversely affecting self-reported quality of life.
Effect on QT interval
QT/QTc measurements were recorded and analysed from a dedicated trial comparing nintedanib monotherapy against sunitinib monotherapy in patients with renal cell carcinoma. In this trial single oral doses of 200 mg nintedanib as well as multiple oral doses of 200 mg nintedanib administered twice daily for 15 days did not prolong the QTcF interval. However, no thorough QT-trial of nintedanib administered in combination with docetaxel was conducted.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Vargatef in all subsets of the paediatric population in non-small cell lung cancer (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Nintedanib reached maximum plasma concentrations approximately 2-4 hours after oral administration as soft gelatin capsule under fed conditions (range 0.5-8 hours). The absolute bioavailability of a 100 mg dose was 4.69% (90% CI: 3.615-6.078) in healthy volunteers. Absorption and bioavailability are decreased by transporter effects and substantial first-pass metabolism. Nintedanib exposure increased dose-proportionally in the dose range of 50-450 mg once daily and 150-300 mg twice daily. Steady state plasma concentrations were achieved within one week of dosing at the latest.
After food intake, nintedanib exposure increased by approximately 20% compared to administration under fasted conditions (CI: 95.3-152.5%) and absorption was delayed (median tmax fasted: 2.00 hours; fed: 3.98 h).
In an in vitro study, mixing nintedanib capsules with a small amount of apple sauce or chocolate pudding for up to 15 minutes did not have any impact on the pharmaceutical quality. Swelling and deformation of the capsules due to the water uptake of the gelatin capsule shell was observed with longer exposure time to the soft food. Therefore, taking the capsules with soft food would not be expected to alter the clinical effect when taken immediately.
Distribution
Nintedanib follows at least bi-phasic disposition kinetics. After intravenous infusion, a high volume of distribution (Vss: 1 050 L, 45.0% gCV) was observed.
The in vitro protein binding of nintedanib in human plasma was high, with a bound fraction of 97.8%. Serum albumin is considered to be the major binding protein. Nintedanib is preferentially distributed in plasma with a blood to plasma ratio of 0.869.
Biotransformation
The prevalent metabolic reaction for nintedanib is hydrolytic cleavage by esterases resulting in the free acid moiety BIBF 1202. BIBF 1202 is subsequently glucuronidated by UGT enzymes, namely UGT 1A1, UGT 1A7, UGT 1A8, and UGT 1A10 to BIBF 1202 glucuronide.
Only a minor extent of the biotransformation of nintedanib consisted of CYP pathways with CYP 3A4 being the predominant enzyme involved. The major CYP-dependent metabolite could not be detected in plasma in the human ADME study. In vitro, CYP-dependent metabolism accounted for about 5% compared to about 25% ester cleavage.
In preclinical in vivo experiments, BIBF 1202 did not show efficacy despite its activity at target receptors of the substance.
Elimination
Total plasma clearance after intravenous infusion was high (CL: 1 390 mL/min, 28.8% gCV). Urinary excretion of the unchanged active substance within 48 h was about 0.05% of the dose (31.5% gCV) after oral and about 1.4% of dose (24.2% gCV) after intravenous administration; the renal clearance was 20 mL/min (32.6% gCV). The major route of elimination of drug related radioactivity after oral administration of [14C] nintedanib was via faecal/biliary excretion (93.4% of dose, 2.61% gCV). The contribution of renal excretion to the total clearance was low (0.649% of dose, 26.3% gCV). The overall recovery was considered complete (above 90%) within 4 days after dosing. The terminal half-life of nintedanib was between 10 and 15 h (gCV% approximately 50%).
Linearity/non-linearity
The pharmacokinetics of nintedanib can be considered linear with respect to time (i.e. single-dose data can be extrapolated to multiple-dose data). Accumulation upon multiple administrations was 1.04-fold for Cmax and 1.38-fold for AUCτ. Nintedanib trough concentrations remained stable for more than one year.
Other information on drug-drug interactions
Metabolism
Drug-drug interactions between nintedanib and CYP substrates, CYP inhibitors, or CYP inducers are not expected, since nintedanib, BIBF 1202, and BIBF 1202 glucuronide did not inhibit or induce CYP enzymes in preclinical studies nor was nintedanib metabolized by CYP enzymes to a relevant extent.
Transport
Nintedanib is a substrate of P-gp. For the interaction potential of nintedanib with this transporter, see section 4.5. Nintedanib was shown to be not a substrate or inhibitor of OATP-1B1, OATP-1B3, OATP-2B1, OCT-2, or MRP-2 in vitro. Nintedanib was also not a substrate of BCRP. Only a weak inhibitory potential on OCT-1, BCRP, and P-gp was observed in vitro which is considered to be of low clinical relevance. The same applies for nintedanib being a substrate of OCT-1.
Pharmacokinetic/pharmacodynamic relationship(s)
In exploratory pharmacokinetic adverse event analyses, higher exposure to nintedanib tended to be associated with liver enzyme elevations, but not with gastrointestinal adverse events. PK-efficacy analyses were not performed for clinical endpoints. Logistic regression revealed a statistically significant association between nintedanib exposure and DCE-MRI response.
Population pharmocokinetic analysis in special populations
The pharmacokinetic properties of nintedanib were similar in healthy volunteers, cancer patients, and patients of the target population. Exposure to nintedanib was not influenced by gender (body weight corrected), mild and moderate renal impairment (estimated by creatinine clearance), liver metastases, ECOG performance score, alcohol consumption, and P-gp genotype.
Population PK analyses indicated moderate effects on exposure to nintedanib depending on age, body weight, and race (see below). Based on the high inter-individual variability of exposure observed in the clinical LUME-Lung-1 trial these effects are not considered clinically relevant. However, close monitoring is recommended in patients with several of these risk factors (see section 4.4).
Age
Exposure to nintedanib increased linearly with age. AUCτ,ss decreased by 16% for a 45-year old patient (5th percentile) and increased by 13% for a 76-year old patient (95th percentile) relative to a patient with the median age of 62 years. The age range covered by the analysis was 29 to 85 years; approximately 5% of the population were older than 75 years.
Body weight
An inverse correlation between body weight and exposure to nintedanib was observed. AUCτ,ss increased by 25% for a 50 kg patient (5th percentile) and decreased by 19% for a 100 kg patient (95th percentile) relative to a patient with the median weight of 71.5 kg.
Race
The population mean exposure to nintedanib was 33-50% higher in Chinese, Taiwanese, and Indian patients and 16% higher in Japanese patients while it was 16-22% lower in Koreans compared to Caucasians (body weight corrected). Based on the high inter-individual variability of exposure these effects are not considered clinically relevant. Data from black individuals was very limited but in the same range as for Caucasians.
Hepatic impairment
In a dedicated single dose phase I trial and compared to healthy subjects, exposure to nintedanib based on Cmax and AUC was 2.2-fold higher in volunteers with mild hepatic impairment (Child Pugh A; 90% CI 1.3-3.7 for Cmax and 1.2-3.8 for AUC, respectively). In volunteers with moderate hepatic impairment (Child Pugh B), exposure was 7.6-fold higher based on Cmax (90% CI 4.4-13.2) and 8.7-fold higher (90% CI 5.7-13.1) based on AUC, respectively, compared to healthy volunteers. Subjects with severe hepatic impairment (Child Pugh C) have not been studied.
Concomitant treatment with oral hormonal contraceptives
In a dedicated pharmacokinetic study, female patients with SSc-ILD received a single dose of a combination of 30 μg ethinylestradiol and 150 μg levonorgestrel before and after twice daily dosing of 150 mg nintedanib for at least 10 days. The adjusted geometric mean ratios (90% confidence interval (CI)) were 117% (108% - 127%; Cmax) and 101% (93% - 111%; AUC0-tz) for ethinylestradiol and 101% (90% - 113%; Cmax) and 96% (91% - 102%; AUC0-tz) for levonorgestrel, respectively (n = 15), indicating that co-administration of nintedanib has no relevant effect on the plasma exposure of ethinylestradiol and levonorgestrel.
Preclinical safety data
General toxicology
Single dose toxicity studies in rats and mice indicated a low acute toxic potential of nintedanib. In repeat dose toxicology studies in rats, adverse effects (e.g. thickening of epiphyseal plates, lesions of the incisors) were mostly related to the mechanism of action (i.e. VEGFR-2 inhibition) of nintedanib. These changes are known from other VEGFR-2 inhibitors and can be considered class effects.
Diarrhoea and vomiting accompanied by reduced food consumption and loss of body weight were observed in toxicity studies in non-rodents.
There was no evidence of liver enzyme increases in rats, dogs, and Cynomolgus monkeys. Mild liver enzyme increases, which were not due to serious adverse effects such as diarrhoea, were only observed in Rhesus monkeys.
Reproduction toxicity
A study of male fertility and early embryonic development to implantation in rats did not reveal effects on the male reproductive tract and male fertility.
In rats, embryofoetal lethality and teratogenic effects were observed at exposure levels below human exposure, at the maximum recommended human dose (MRHD) of 200 mg b.i.d. Effects on the development of the axial skeleton and on the development of the great arteries were also noted at subtherapeutic exposure levels.
In rabbits, embryofoetal lethality was observed at an exposure approximately 8 times higher than at the MRHD. Teratogenic effects on the aortic arches in combination with the heart and the urogenital system were noted at an exposure 4 times higher than at the MRHD and on the embryofoetal development of the axial skeleton at an exposure 3 times higher than at the MRHD.
In rats, small amounts of radiolabelled nintedanib and/or its metabolites were excreted into the milk (≤0.5% of the administered dose).
Genotoxicity studies indicated no mutagenic potential for nintedanib.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.