VEOPOZ Solution for injection Ref.[107419] Active ingredients: Pozelimab
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
Pozelimab-bbfg is a human, monoclonal immunoglobulin G4P (IgG4P) antibody directed against the terminal complement protein C5 that inhibits terminal complement activation by blocking cleavage of C5 into C5a (anaphylatoxin) and C5b, thereby blocking the formation of the membrane-attack complex (C5b-C9, a structure mediating cell lysis).
12.2. Pharmacodynamics
The effect of pozelimab-bbfg on complement activity was measured by total complement hemolytic activity test (CH50). The magnitude and duration of reduction from baseline in CH50 by pozelimab-bbfg was dose dependent.
In healthy subjects receiving a single dose of VEOPOZ 30 mg/kg administered as an intravenous infusion over approximately one hour, the complete inhibition of CH50 was achieved at the end of infusion in all subjects, was maintained for 28 days, and returned to baseline 84 days post-dose. After a single dose of VEOPOZ administered as a 600 mg subcutaneous injection, the maximum reduction from baseline in CH50 was achieved 7 days post-dose in most subjects (range: 3 to 14 days), corresponding to Tmax, and returned to baseline 56 days post-dose.
In patients with CD55-deficient PLE receiving a single 30 mg/kg dose administered as an intravenous infusion over approximately one hour followed by a weight-tiered subcutaneous injection once weekly starting at Week 1, CH50 was completely inhibited by Week 1 for most subjects and by Week 12 for all patients.
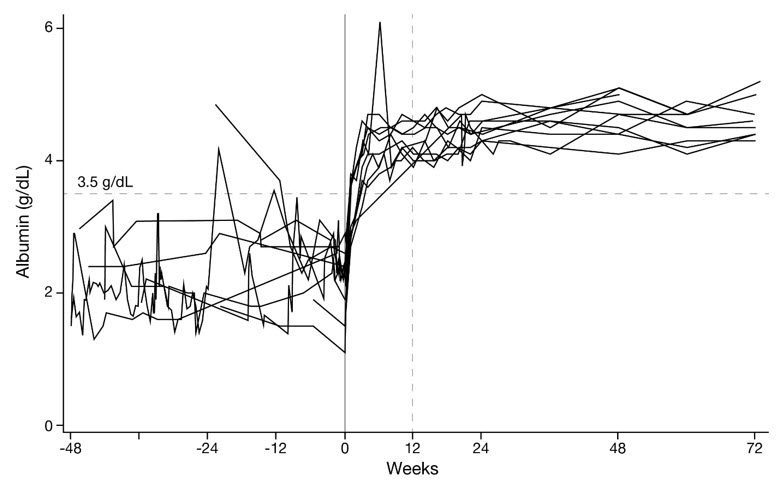
In the same study of patients with CD55-deficient PLE, serum albumin concentrations increased as early as Week 1 and reached the normal range (≥3.5 g/dL) by Week 4 for most subjects and by Week 12 for all subjects. The serum albumin concentrations were maintained within the normal range for the duration of treatment. Endogenous serum IgG concentrations were also increased from baseline at Week 1 in all patients with CD55-deficient PLE and reached a stable concentration around Week 16 [see Clinical Studies (14)].
12.3. Pharmacokinetics
In healthy subjects, single intravenous infusions of VEOPOZ over approximately one hour resulted in dose proportional increases in mean Cmax, but greater than proportional increases in mean AUCinf (>16-fold) for total pozelimab concentrations in serum between 3 mg/kg and 30 mg/kg. The mean AUCinf increased by 3.5-fold between 10 mg/kg and 30 mg/kg. In healthy subjects, single subcutaneous injections of VEOPOZ resulted in approximately 1.5-fold increase in mean Cmax and 2.2-fold increase in mean AUCinf between 300 mg and 600 mg.
In patients with CD55-deficient PLE, a single dose of VEOPOZ 30 mg/kg administered as an intravenous infusion over approximately one hour resulted in median (range) total pozelimab trough concentration of 180 (52.8, 268) mg/L at Week 1. The predicted mean (SD) trough concentrations of total pozelimab at steady state are 330 (94.2) mg/L and 385 (112) mg/L for VEOPOZ 10 mg/kg or 12 mg/kg (up to a maximum 800 mg) once weekly via subcutaneous injection(s), respectively, following the intravenous loading dose.
Absorption
In healthy subjects, following subcutaneous injection of 600 mg, the bioavailability of pozelimab-bbfg is estimated as 51%. The median (range) time to reach peak concentration was 7 (3 to 7) days following a single subcutaneous injection of 300 mg or 600 mg in healthy subjects.
Distribution
In healthy adult subjects with a mean body weight of 70 kg, the mean (SD) volume of distribution following a single intravenous dose of 30 mg/kg was 3.3 (0.4) L. The mean (SD) apparent volume of distribution following a single subcutaneous injection of 300 mg and 600 mg was 6.0 (0.9) L and 8.6 (2.7) L, respectively.
Elimination
Pozelimab-bbfg is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG. Pozelimab-bbfg elimination is mediated via linear and non-linear pathways. At higher concentrations, pozelimab-bbfg elimination is primarily through the linear non-saturable proteolytic pathway, whereas at lower concentrations, the non-linear, saturable C5 target-mediated elimination predominates.
In healthy adult subjects, the median (range) terminal half-life of total pozelimab in serum was 13.5 (10.0, 17.2) days following a single 30 mg/kg dose administered as an intravenous infusion. The median (range) terminal half-life was 14.1 (8.6, 17.3) days following a single 600 mg subcutaneous injection.
In patients with CD55-deficient PLE, steady-state total pozelimab concentrations were reached at approximately 20 weeks following subcutaneous injection once weekly.
Specific Populations
Body Weight
Body weight was a significant covariate on the pharmacokinetics of pozelimab-bbfg.
Renal and Hepatic Impairment
Pozelimab-bbfg, a monoclonal antibody, is not likely to undergo renal or hepatic excretion.
Drug Interaction Studies
Drug interaction studies have not been conducted. Intravenous Immunoglobulin (IVIg) may interfere with the endosomal neonatal Fc receptor (FcRn) recycling mechanism of monoclonal antibodies such as pozelimab-bbfg, which may decrease serum pozelimab concentrations [see Drug Interactions (7.1)].
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Carcinogenicity studies have not been conducted with pozelimab-bbfg. The mutagenic potential of pozelimab-bbfg has not been evaluated; however, monoclonal antibodies are not expected to alter DNA or chromosomes.
Impairment of Fertility
Fertility studies have not been conducted with pozelimab-bbfg. In a 6-month toxicity study in sexually-mature male and female monkeys, pozelimab-bbfg had no adverse effects on histological or functional markers of reproductive function (e.g., estrous cyclicity, testicular volume, ejaculate amount, total sperm count per ejaculate, sperm motility and morphology, and histology of reproductive organs) at doses up to 100 mg/kg/week (11.9 to 13.9-fold the predicted exposures at the recommended clinical doses, on an AUC basis).
14. Clinical Studies
The efficacy and safety of VEOPOZ were evaluated in a single-arm study (NCT04209634) where outcomes were compared to pre-treatment data in patients with active CD55-deficient protein-losing enteropathy (PLE) who had hypoalbuminemia. Diagnosis was based on a clinical history of PLE and with a confirmed genotype of biallelic CD55 loss-of-function mutation.
Active CD55-deficient PLE was defined as hypoalbuminemia (serum albumin concentration of ≤3.2 g/dL) with one or more of the following signs or symptoms within the last six months: abdominal pain, diarrhea, peripheral edema, or facial edema.
Patients received a single 30 mg/kg loading dose of VEOPOZ administered by intravenous infusion over approximately one hour, followed by a once weekly weight-tiered maintenance dosage, administered as a subcutaneous injection starting one week after the loading dose.
All patients received meningococcal vaccination prior to treatment with VEOPOZ and antibacterials for prophylaxis of meningococcal infection. Patients were permitted to receive additional therapies as part of standard of care. Use of other complement inhibitors was prohibited.
Ten patients ranging from 3 to 19 years of age (median of 8.5 years) were assessed for efficacy. Six patients identified as female; seven patients as White, two patients as Asian, and one patient reported race as other. The mean baseline serum albumin concentration was 2.2 g/dL with a range of 1.1 to 2.9 g/dL.
Additional Efficacy Results
Serum IgG concentrations reached normal values for age in all patients within the first 12 weeks of treatment; improvement was maintained through at least 72 weeks of treatment.
Serum Albumin Concentrations
The median time for serum albumin to reach at least 3.5 g/dL was 15.5 days (N=10; 95% CI: 8 to 28). All 10 patients achieved normalization by Week 12 and maintained serum albumin concentrations within the normal range through at least 72 weeks of treatment (Figure 1).
Figure 1. Serum Albumin Concentrations from 48 Weeks Pre-Treatment Through 72 Weeks on VEOPOZ in Ten Patients with CD55-Deficient PLE:
Albumin Transfusions
Five of the 10 patients received a total of 60 transfusions in the 48 weeks prior to treatment. In the 48 weeks after starting treatment, one patient received one albumin transfusion.
Hospitalizations
Nine of the 10 patients were hospitalized for a total of 268 days in the 48 weeks prior to treatment. In the 48 weeks after starting treatment, two patients were hospitalized for a total of 7 days.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.