VERASEAL Solutions for sealant Ref.[28242] Active ingredients: Human fibrinogen Thrombin
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Instituto Grifols, S.A., Can Guasc, 2 - Parets del Vallès, E-08150 Barcelona - Spain
4.1. Therapeutic indications
Supportive treatment in adults where standard surgical techniques are insufficient:
- for improvement of haemostasis.
- as suture support: in vascular surgery.
4.2. Posology and method of administration
The use of VeraSeal is restricted to experienced surgeons who have been trained in the use of this medicinal product.
Posology
The volume of VeraSeal to be applied and the frequency of application should always be oriented towards the underlying clinical needs for the patient.
The dose to be applied is governed by variables including, but not limited to, the type of surgical intervention, the size of the area and the mode of intended application, and the number of applications.
Application of the product must be individualised by the treating physician. In clinical trials, the individual dosages have typically ranged from 0.3 to 12 ml. For other procedures, larger volumes may be required.
The initial volume of the product to be applied at a chosen anatomic site or target surface area should be sufficient to entirely cover the intended application area. The application can be repeated, if necessary.
Paediatric population
The safety and efficacy of VeraSeal in children aged 0 to 18 years has not yet been established. Currently available data are described in section 5.1, but no recommendation on a posology can be made.
Method of administration
For epilesional use.
For instructions on preparation of the medicinal product before administration, see section 6.6. The product should only be administered according to the instructions and with the devices recommended for this product (see section 6.6.).
Prior to applying VeraSeal, the surface area of the wound needs to be dried by standard techniques (e.g. intermittent application of compresses, swabs, use of suction devices).
For spray application, see sections 4.4 and 6.6 for specific recommendations on the required distance from tissue per surgical procedure.
4.9. Overdose
In the event of overdose, patients must be closely monitored for signs or symptoms of adverse reactions and appropriate symptomatic treatment and supportive measures instituted.
6.3. Shelf life
2 years.
After thawing, it can be maintained not more than 48 hours at 2ºC-8ºC or 24 hours at room temperature (20ºC-25ºC) before use if it remains sealed in the original packaging.
In use shelf life: Once the blister is opened, VeraSeal should be used immediately.
6.4. Special precautions for storage
Store and transport in a freezer (at -18ºC or colder). The cold storage chain (-18ºC or colder) must not be interrupted until use. Keep the sterilized blister in the outer carton to protect from light.
Once thawed, do not refreeze. For storage conditions after thawing the medicinal product and after first opening, see section 6.3.
6.5. Nature and contents of container
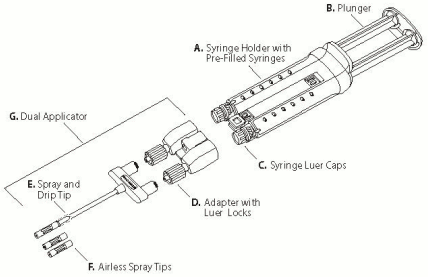
VeraSeal is supplied as a single-use kit containing two pre-filled syringes (glass type I) with rubber stoppers, each with a sterile frozen solution, assembled in a syringe holder.
One Dual Applicator with two additional Airless Spray Tips is supplied with the product, for application by spraying or dripping. The Airless Spray Tips are radiopaque. See scheme below.
VeraSeal is available in the following pack sizes:
- VeraSeal 2 ml (containing 1 ml of human fibrinogen and 1 ml of human thrombin)
- VeraSeal 4 ml (containing 2 ml of human fibrinogen and 2 ml of human thrombin)
- VeraSeal 6 ml (containing 3 ml of human fibrinogen and 3 ml of human thrombin)
- VeraSeal 10 ml (containing 5 ml of human fibrinogen and 5 ml of human thrombin)
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
The instructions for use are also described in the healthcare professionals' package leaflet part.
Remove carton from freezer, open it and take out the two blisters.
Place the blister containing the Dual Applicator at room temperature until the fibrin sealant is ready to use.
Room temperature thawing (preferred method)
Thaw blister with VeraSeal pre-filled syringes at room temperature using the following steps:
1. Place the blister containing the syringe holder with pre-filled syringes on a surface at room temperature (20ºC–25ºC)
- for approximately 70 minutes for the 2 ml and the 4 ml package sizes
- for approximately 90 minutes for the 6 ml and the 10 ml package sizes
After thawing, it is not necessary to warm the product for its use.
After thawing the solutions must be clear to slightly opalescent and colourless to pale yellow. Solutions that are cloudy or have deposits should not be used.
Post-Thawing Storage:
After thawing, the kit containing the VeraSeal syringe holder with pre-filled syringes and Dual Applicator can be stored before use for not more than 48 hours in the refrigerator at 2–8ºC or 24 hours at room temperature (20-25°C) if it remains sealed in the original packaging. Once the blisters are opened, use VeraSeal immediately and discard any unused contents.
Once thawed, do not refreeze.
Transferring instructions:
1. After thawing, remove the blister from surface at room temperature or from the refrigerator at 2°C-8°C.
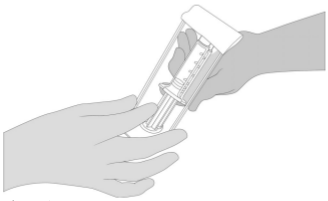
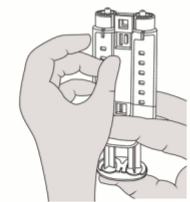
2. Open the blister and make the VeraSeal syringe holder with pre-filled syringes available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field. See Figure 1.
Figure 1:
Sterile Water Bath (Quick Thawing)
Thaw VeraSeal pre-filled syringes inside the sterile field in a sterile thermostatic water bath at a temperature not higher than 37ºC using the following steps:
NOTE: Once the VeraSeal blisters are opened, use the product immediately. Use sterile technique to avoid the possibility of contamination due to improper handling, and follow the steps below accurately. Do not remove the syringe luer cap until thawing is complete and the Dual Applicator is ready to be attached.
1. Open the blister and make the VeraSeal syringe holder with pre-filled syringes available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field. See Figure 1.
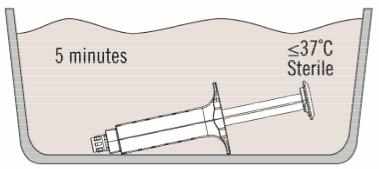
2. Place the syringe holder with pre-filled syringes directly into the sterile water bath ensuring that it is completely immersed in the water. See Figure 2.
3. At 37ºC, the time needed is approximately 5 minutes for the 2 ml, 4 ml, 6 ml, and 10 ml package sizes, but must not be left at this temperature for longer than 10 minutes. The temperature of the water bath must not exceed 37ºC.
4. Dry the syringe holder with pre-filled syringes after thawing, using a sterile surgical gauze.
Figure 2:
After thawing, the solutions must be clear to slightly opalescent and colorless to pale yellow. Do not use solutions that are cloudy or have deposits.
Use VeraSeal immediately and discard any unused contents.
Connection instructions
1. Open the blister and make the VeraSeal Dual Applicator and two additional Airless Spray Tips available to a second person for transfer to the sterile field. The outside of the blister should not come in contact with the sterile field.
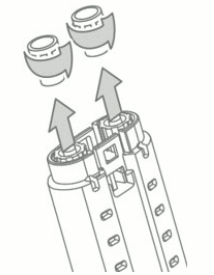
2. Hold the VeraSeal syringe holder with syringe luer caps pointed upward. See Figure 3.
3. Unscrew and discard the syringe luer cap of both fibrinogen and thrombin syringes. See Figure 3.
Figure 3:
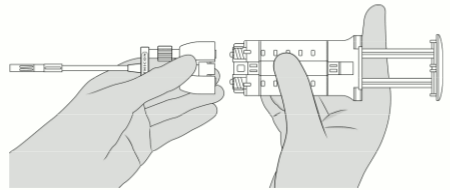
4. Hold the syringe holder with the luers pointed upward. To remove air bubbles from syringes, strike gently the side of the syringe holder one or two times while keeping the syringe holder in an upright position and lightly depress the plunger to eject air. See Figure 4.
Figure 4:
5. Attach the Dual Applicator. See Figure 5.
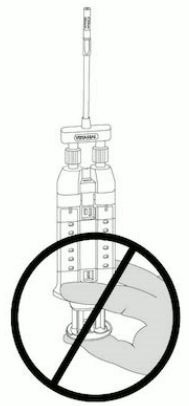
NOTE: Do not depress plunger during attachment or prior to intended use because the two biologic components will pre-mix in the Airless Spray Tip, forming a fibrin clot that prevents dispensing. See Figure 6.
Figure 5:
Figure 6:
6. Tighten luer locks and ensure the Dual Applicator is firmly attached. The device is now ready to use.
Administration
Apply VeraSeal using the syringe holder and plunger supplied.
Apply VeraSeal using the Dual Applicator provided with the product. Other CE-marked applicator tips (including open surgery and laparoscopic use devices) intended for specific use with VeraSeal may also be used. When using the provided Dual Applicator, follow the connection instructions described above. When using other applicator tips, follow the instructions for use that are provided with the applicator tips.
Application by spraying:
1. Grasp and bend the Dual Applicator to the desired position. Tip will retain its shape.
2. Position the Airless Spray Tip at least 2 cm away from the target tissue. Apply firm even pressure to the plunger to spray the fibrin sealant. Increase distance accordingly to achieve desired coverage of the target area.
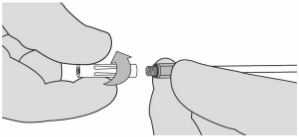
3. If expression is stopped for any reason, change the Airless Spray Tip. To change the Airless Spray Tip, remove the device from the patient and unscrew the used Airless Spray Tip. See Figure 7. Place the used Airless Spray Tip away from the spare Airless Spray Tips. Wipe the end of the applicator using dry or moist sterile surgical gauze. Then, connect a new Airless Spray Tip provided in the package and ensure it is firmly connected before use.
NOTE: Red indicator will not be visible if Airless Spray Tip is properly connected. See Figure 8.
NOTE: Do not continue pushing the plunger in an attempt to clear the fibrin clot within the Airless Spray Tip; otherwise the applicator may become unusable.
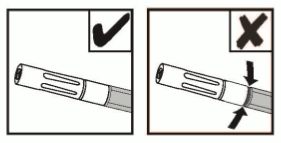
NOTE: Do not trim the Dual Applicator to avoid exposing internal wire.
Figure 7:
Figure 8:
Application by dripping:
1. Remove the Airless Spray Tip portion of the spray and drip tip by unscrewing the Airless Spray Tip. See Figure 7.
2. Grasp and bend the drip tip to the desired position. Tip will retain its shape.
3. During dripping, keep the end of the drip tip as close to the tissue surface as possible without touching the tissue during application.
4. Apply individual drops to the surface area to be treated. To prevent uncontrolled clotting, allow the drops to separate from each other and from the end of the drip tip.
NOTE: Do not reconnect a used drip tip after it has been removed from the adapter; otherwise a clot may form inside the drip tip and the applicator may become unusable.
Disposal
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.