VEYVONDI Powder and solvent for solution for injection Ref.[27668] Active ingredients: Von Willebrand factor
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Baxalta Innovations GmbH, Industriestraße 67, 1221 Vienna, Austria
4.1. Therapeutic indications
VEYVONDI is indicated in adults (age 18 and older) with von Willebrand Disease (VWD), when desmopressin (DDAVP) treatment alone is ineffective or not indicated for the
- Treatment of haemorrhage and surgical bleeding.
- Prevention of surgical bleeding.
VEYVONDI should not be used in the treatment of Haemophilia A.
4.2. Posology and method of administration
Treatment of von Willebrand disease (VWD) should be supervised by a physician experienced in the treatment of haemostatic disorders.
Posology
Dosage and frequency of administration must be individualized according to clinical judgement and based on the patient´s weight, type and severity of the bleeding episodes/surgical intervention and based on monitoring of appropriate clinical and laboratory measures. Dose based on bodyweight may require adjustment in underweight or overweight patients.
Generally, 1 IU/kg (VWF:RCo/VEYVONDI/vonicog alfa) raises the plasma VWF:RCo by 0.02 IU/mL (2%).
Haemostasis cannot be ensured until Factor VIII coagulant activity (FVIII:C) is at least 0.4 IU/mL (≥40% of normal activity). Depending on the patient's baseline FVIII:C levels, a single infusion of rVWF will, in a majority of patients, lead to an increase above 40% in endogenous FVIII:C activity within 6 hours and will result in sustaining this level up to 72 hours post infusion. The dose and duration of the treatment depend on the clinical status of the patient, the type and severity of the bleeding, and both VWF:RCo and FVIII:C levels. If the patient's baseline plasma FVIII:C level is < 40% or is unknown and in all situations where a rapid correction of haemostasis should be achieved, such as treatment of an acute haemorrhage, severe trauma or emergency surgery, it is necessary to administer a recombinant factor VIII product with the first infusion of VEYVONDI, in order to achieve a haemostatic plasma level of FVIII:C.
However, if an immediate rise in FVIII:C is not necessary, or if the baseline FVIII:C level is sufficient to ensure haemostasis, the physician may decide to omit the co-administration of rFVIII at the first infusion with VEYVONDI.
In case of major bleeding events or major surgeries requiring repeated, frequent infusions, monitoring of FVIII:C levels is recommended, to decide if rFVIII is required for subsequent infusions to avoid excessive rise of FVIII:C.
Treatment of bleeding episodes (On demand treatment)
Start of treatment
The first dose of VEYVONDI should be 40 to 80 IU/kg body weight. Replacement levels of VWF:RCo >0.6 IU/mL (60%) and FVIII:C >0.4 IU/mL (40%) should be achieved. Dosing guidelines for treatment of minor and major haemorrhages are provided in Table 1.
VEYVONDI should be administered with recombinant factor VIII if the FVIII:C levels are <40%, or are unknown, to control bleeding. The rFVIII dose should be calculated according to the difference between the patient's baseline plasma FVIII:C level, and the desired peak FVIII:C level to achieve an appropriate plasma FVIII:C level based on the approximate mean recovery of 0.02 (IU/mL)/(IU/kg). The complete dose of VEYVONDI should be administered followed by rFVIII within 10 minutes.
Calculating dose:
VEYVONDI dose [IU] = dose [IU/kg] x weight [kg]
Subsequent infusions:
A subsequent dose of 40 IU to 60 IU/kg of VEYVONDI should be infused every 8 to 24 hours as per the dosing ranges in Table 1, or as long as clinically appropriate. In major bleeding episodes, maintain trough levels of VWF:RCo greater than 50% for as long as deemed necessary.
Based on experience from clinical studies, once VWF has been replaced, endogenous FVIII levels will remain normal or near normal as long as VEYVONDI is continued to be administered.
Table 1. Dosing recommendations for the treatment of minor and major haemorrhages:
| Haemorrhage | Initial dosea (IU VWF:RCo/kg body weight) | Subsequent dose |
|---|---|---|
| Minor (e.g. epistaxis, oral bleeding, menorrhagia) | 40 to 50 IU/kg | 40 to 50 IU/kg every 8 to 24 hours (or as long as deemed clinically necessary) |
| Majorb (e.g. severe or refractory epistaxis, menorrhagia, gastrointestinal bleeding, central nervous system trauma, haemarthrosis, or traumatic haemorrhage) | 50 to 80 IU/kg | 40 to 60 IU/kg every 8 to 24 hours for approximately 2-3 days (or as long as deemed clinically necessary) |
a If rFVIII is administered, see rFVIII package insert for reconstitution and administration instructions.
b A bleed could be considered major if red blood cell transfusion is either required or potentially indicated or if bleeding occurs in a critical anatomical site (e.g., intracranial or gastrointestinal haemorrhage).
Prevention of bleeding/haemorrhage and treatment in case of elective surgery
Prior to Surgery:
In patients with inadequate levels of FVIII, a dose of 40-60 IU/kg VEYVONDI should be administered 12-24 hours prior to initiating elective surgery (pre-operative dose), to ensure pre-operative endogenous FVIII levels of at least 0.4 IU/mL for minor and at least 0.8 IU/mL for major surgery.
For prevention of excessive bleeding in case of elective surgery, within 3 hours prior to initiation of any surgical procedure, the FVIII:C levels should be assessed. If the FVIII:C levels are at the recommended target level of:
- at least 0.4 IU/mL for minor and oral surgery and
- at least 0.8 IU/mL for major surgery,
a dose of VEYVONDI alone should be administered within 1 hour prior to the procedure.
If the FVIII:C levels are not at the recommended target levels, rFVIII should be administered in addition to vonicog alfa to raise VWF:RCo and FVIII:C, within 1 hour prior to the procedure. Please refer to Table 2 for FVIII:C recommended target levels. The dose depends on VWF and FVIII levels of the patient, the type and severity of the expected bleeding.
Table 2. Recommended Target Peak Plasma Levels of VWF:RCo and FVIII:C to be Achieved Prior to Surgery for the Prevention of Excessive Bleeding During and After Surgery:
|<.Type of Surgery|<>.VWF:RCo Target Peak Plasma Level|<>.FVIII:C Target Peak Plasma Levela |<>.Calculation of rVWF dose (to be administered within 1 hour prior to surgery) (IU VWF:RCo required) |
|Minor|<>0.50–0.60 IU/mL |<>0.40 – 0.50 IU/mL |<>∆b VWF:RCo x BW (kg)/IRc |
|Major|<>1 IU/mL|<>0.80 - 1 IU/mL |<>∆b VWF:RCo x BW (kg)/IRc |
a Additional rFVIII may be required to attain the recommended FVIII:C target peak plasma levels. Dosing guidance should be done based on the IR.
b ∆ = Target peak plasma VWF:RCo – baseline plasma VWF:RCo
c IR = Incremental Recovery as measured in the subject. If the IR is not available, assume an IR of 0.02 IU/mL per IU/kg.
During and After Surgery:
After the initiation of the surgical procedure, the VWF:RCo and FVIII:C plasma levels should be monitored and the intra- and post-operative substitution regimen should be individualised according to the PK results, intensity and duration of the haemostatic challenge, and the institution's standard of care. In general, the frequency of VEYVONDI dosing for post-operative substitution should range from twice a day to every 48 hours. Please refer to Table 3 for treatment recommendations for subsequent maintenance doses.
Table 3. Recommended Target Trough Plasma Levels of VWF:RCo and FVIII:C and Minimum Duration of Treatment for Subsequent Maintenance Doses for the Prevention of Excessive Bleeding After Surgery:
| Type of Surgery | VWF:RCo Target Trough Plasma Level | FVIII:C Target Trough Plasma Level | Minimum Duration of treatment | Frequency of dosing | ||
|---|---|---|---|---|---|---|
| Up to 72 hours post surgery | After 72 hours post surgery | Up to 72 hours post surgery | After 72 hours post surgery | |||
| Minor | ≥0.30 IU/mL | - | >0.40 IU/mL | - | 48 hours | Every 12-24 hrs / every other day |
| Major | >0.50 IU/mL | >0.30 IU/mL | >0.50 IU/mL | >0.40 IU/mL | 72 hours | Every 12-24 hrs / every other day |
Paediatric population
The safety and efficacy of VEYVONDI in children aged 0 to 18 years have not yet been established. No data are available.
Method of administration
VEYVONDI is for intravenous use. The reconstituted product should be inspected visually prior to administration.
The rate of administration should be slow enough to ensure the comfort of the patient, up to a maximum of 4 mL/min. The patient should be observed for any immediate reaction. If any reaction, such as tachycardia, occurs that might be related to the administration of the product, the rate of infusion should be reduced or stopped as required by the clinical condition of the patient. When co-administration of rVWF and rFVIII is considered necessary, they can be pre-mixed in a single syringe to achieve the appropriate dose. The contents of each vial of rVWF and rFVIII can be drawn into a single syringe by using a separate unused reconstitution device (see 6.2 for incompatibilities).
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
4.9. Overdose
No symptoms of overdose with von Willebrand factor have been reported. Thromboembolic events may occur in case of major overdose.
6.3. Shelf life
Unopened vial:
3 years.
Shelf-life after reconstitution:
Chemical and physical in-use stability has been demonstrated for 3 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless reconstitution has taken place in controlled and validated aseptic conditions.
6.4. Special precautions for storage
Powder:
Do not store above 30°C.
Do not freeze.
Store in the original package in order to protect from light.
After reconstitution:
For storage conditions after reconstitution of the medicinal product, see section 6.3.
6.5. Nature and contents of container
VEYVONDI 650 IU powder and solvent for solution for injection
Each pack contains:
- powder in a vial (type I glass), with a butyl rubber stopper
- 5 mL of solvent in a vial (type I glass), with a rubber stopper (chlorobutyl)
- one reconstitution device (Mix2Vial)
VEYVONDI 1300 IU powder and solvent for solution for injection
Each pack contains:
- powder in a vial (type I glass), with a butyl rubber stopper
- 10 mL of solvent in a vial (type I glass), with a rubber stopper (bromobutyl)
- one reconstitution device (Mix2Vial)
6.6. Special precautions for disposal and other handling
General Instructions:
- Check the expiry date, and ensure that the VEYVONDI powder and water for injections (solvent) are at room temperature prior to preparation. Do not use after the expiry date stated on the labels and carton.
- Use antiseptic technique (clean and low-germ conditions) and a flat work surface during the reconstitution procedure. Wash your hands and put on clean exam gloves (the use of gloves is optional).
- Use the reconstituted product (after mixing the powder with the supplied water) as soon as possible and within three hours. You can store the reconstituted product at room temperature not to exceed 25°C for up to three hours.
- Ensure that the VEYVONDI powder vial and the sterilised water for injections (solvent) are at room temperature prior to preparation.
- Use plastic syringes with this product because proteins in the product tend to stick to the surface of glass syringes.
- Do not mix vonicog alfa with other medicinal products except for rFVIII.
Instructions for Reconstitution and application:
| Steps | Image example | |
|---|---|---|
| 1 | Remove the caps from the VEYVONDI powder and solvent vials to expose the center of the rubber stoppers. | 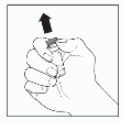 |
| 2 | Disinfect each stopper with a separate sterile alcohol swab (or other suitable sterile solution suggested by your doctor or haemophilia treatment center) by wiping the stopper for several seconds. Allow the rubber stopper to dry. Place the vials on a flat surface. |  |
| 3 | Open the Mix2Vial device package by completely peeling away the lid, without touching the inside of the package. Do not remove the Mix2Vial device from the package. | NA |
| 4 | Turn the package with the Mix2Vial device upside down and place it over the top of the solvent vial. Firmly insert the blue plastic spike of the device into the center of the solvent vial stopper by pushing straight down. Grip the package at its edge and lift it off the Mix2Vial device. Be careful not to touch the clear plastic spike. The solvent vial now has the Mix2Vial device connected to it and is ready to be connected to the VEYVONDI vial. | 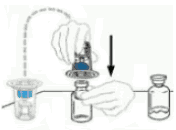 |
| 5 | To connect the solvent vial to the VEYVONDI vial, turn the solvent vial over and place it on top of the vial containing VEYVONDI powder. Fully insert the clear plastic spike into the VEYVONDI vial stopper by firmly pushing straight down. This should be done right away to keep the liquid free of germs. The solvent will flow into the VEYVONDI vial by vacuum. Check that all the solvent has transferred. Do not use if the vacuum has been lost and the solvent does not flow into the VEYVONDI vial. | 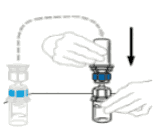 |
| 6 | Gently and continuously swirl the connected vials or allow the reconstituted product to stand for 5 minutes then gently swirl to ensure the powder is completely dissolved. Do not shake. Shaking will adversely affect the product. Do not refrigerate after reconstitution. | 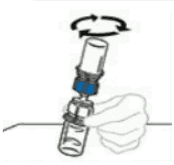 |
| 7 | Disconnect the two sides of the Mix2Vial from each other by holding the clear plastic side of the Mix2Vial device attached to the VEYVONDI vial with one hand and the blue plastic side of the Mix2Vial device attached to the solvent vial with the other hand. Turn the blue plastic side counterclockwise and gently pull the two vials apart. Do not touch the end of the plastic connector attached to the VEYVONDI vial containing the dissolved product. Place the VEYVONDI vial on a flat work surface. Discard the empty solvent vial. | 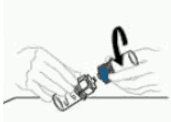 |
| 8 | Draw air into the empty, sterile disposable plastic syringe by pulling back on the plunger. The amount of air should equal the amount of reconstituted VEYVONDI that you will withdraw from the vial. | 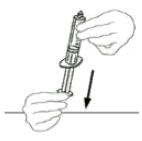 |
| 9 | Leaving the VEYVONDI vial (containing the reconsituted product) on your flat work surface, connect the syringe to the clear plastic connector and turning the syringe clockwise. | 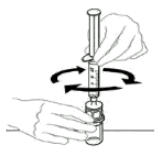 |
| 10 | Hold the vial with one hand and use the other hand to push all the air from the syringe into the vial. | 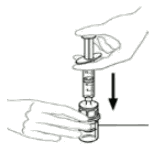 |
| 11 | Flip connected syringe and VEYVONDI vial so the vial is on top. Be sure to keep the syringe plunger pressed in. Draw the VEYVONDI into the syringe by pulling plunger back slowly. |  |
| 12 | Do not push and pull solution back and forth between syringe and vial. Doing so may harm medicine. When ready to infuse, disconnect the syringe by turning it counterclockwise. Inspect the syringe visually for particulate matter; the solution should be clear and colourless. If flakes or particles are seen, do not use the solution and notify your doctor. |  |
| 13 | If you need more than one vial of VEYVONDI to make up your dose: • Leave the syringe attached to the vial until an additional vial is prepared. • Use the reconstitution steps above (2 to 8) to prepare the additional vial of VEYVONDI using a fresh Mix2Vial device for each vial. | |
| 14 | The contents of two vials may be drawn into a single syringe. |
NOTE: When pushing air into a second vial of VEYVONDI to be pooled into a syringe, orient the vial and connected syringe with the vial on top.
Instructions for Administration:
Inspect the prepared solution in the syringe for particulate matter and discoloration prior to administration (the solution should be clear, colourless and free from particles). It is not uncommon for a few flakes or particles to remain in the product vial after reconstitution. The filter included in the Mix2Vial device removes those particles completely. Filtration does not influence dosage calculations. The solution in the syringe should not be used if it is cloudy or contains flakes or particles after filtration.
1. Attach the infusion needle to a syringe containing VEYVONDI solution. For comfort, a winged (butterfly) infusion set is preferred. Point the needle up and remove any air bubbles by gently tapping the syringe with your finger and slowly and carefully pushing air out of the syringe and needle.
2. Apply a tourniquet and get the infusion site ready by wiping the skin well with a sterile alcohol swab (or other suitable sterile solution suggested by your doctor or haemophilia treatment center).
3. Insert the needle into the vein and remove the tourniquet. Slowly infuse VEYVONDI. Do not infuse any faster than 4 mL per minute. Disconnect the empty syringe. If your dose requires multiple syringes, attach and administer each additional syringe of VEYVONDI one at a time.
Note:
Do not remove butterfly needle until all syringes have been infused and do not touch the Luer port that connects to the syringe.
If recombinant factor VIII has been prescribed, administer recombinant factor VIII within 10 minutes after infusion of VEYVONDI has been completed.
4. Take the needle out of the vein and use sterile gauze to put pressure on the infusion site for several minutes.
In case large volumes of VEYVONDI are required, it is possible to pool two vials of VEYVONDI together. The contents of each reconstituted product of VEYVONDI can be drawn in a single syringe. However, in these cases the initially reconstituted solution of VEYVONDI should not be diluted any further.
The solution should be slowly administered intravenously (see section 4.2) not exceeding 4 mL/min.
Do not recap the needle. Place the needle, syringe, and empty VEYVONDI and solvent vial(s) in a hard-walled sharps container for proper disposal. Do not dispose of these supplies in ordinary household trash.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.