VIEKIRAX Film-coated tablet Ref.[108064] Active ingredients: Ombitasvir Ombitasvir, Paritaprevir and Ritonavir Paritaprevir Ritonavir
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: AbbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Patients with moderate to severe hepatic impairment (Child-Pugh B or C) (see section 5.2).
Use of ethinyloestradiol-containing medicinal products such as those contained in most combined oral contraceptives or contraceptive vaginal rings (see sections 4.4 and 4.5).
Medicinal products that are highly dependent on CYP3A for clearance and for which elevated plasma levels are associated with serious events must not be co-administered with Viekirax (see section 4.5). Examples are provided below.
CYP3A4 substrates:
- alfuzosin hydrochloride
- amiodarone, disopyramide, dronedarone, quinidine, ranolazine
- astemizole, terfenadine
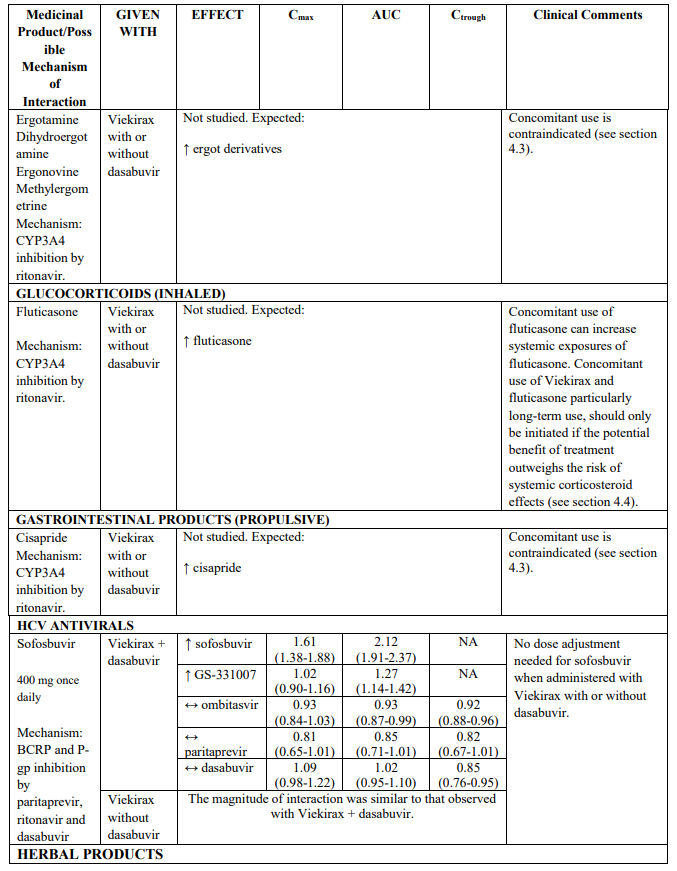
- cisapride
- colchicine in patients with renal or hepatic impairment
- ergotamine, dihydroergotamine, ergonovine, methylergometrine
- fusidic acid
- lomitapide
- lovastatin, simvastatin, atorvastatin
- lurasidone
- oral midazolam, triazolam
- pimozide
- quetiapine
- salmeterol
- sildenafil (when used for the treatment of pulmonary arterial hypertension)
- ticagrelor
Co-administration of Viekirax with or without dasabuvir with medicinal products that are strong or moderate enzyme inducers is expected to decrease ombitasvir, paritaprevir, and ritonavir plasma concentrations and reduce their therapeutic effect and must not be co-administered (see section 4.5). Examples of contraindicated strong or moderate enzyme inducers are provided below.
Enzyme inducers:
- carbamazepine, phenytoin, phenobarbital
- efavirenz, nevirapine, etravirine
- apalutamide,enzalutamide
- mitotane
- rifampicin
- St. John’s Wort (Hypericum perforatum)
Co-administration of Viekirax with or without dasabuvir with medicinal products that are strong inhibitors of CYP3A4 is expected to increase paritaprevir plasma concentrations and must not be co-administered with Viekirax (see section 4.5). Examples of contraindicated strong CYP3A4 inhibitors are provided below.
CYP3A4 inhibitors:
- cobicistat
- indinavir, lopinavir/ritonavir, saquinavir, tipranavir,
- itraconazole, ketoconazole, posaconazole, voriconazole
- clarithromycin, telithromycin
- conivaptan
4.4. Special warnings and precautions for use
General
Viekirax is not recommended for administration as monotherapy and must be used in combination with other medicinal products for the treatment of hepatitis C infection (see sections 4.2 and 5.1).
Risk of hepatic decompensation and hepatic failure in patients with cirrhosis
Hepatic decompensation and hepatic failure, including liver transplantation or fatal outcomes, have been reported postmarketing in patients treated with Viekirax with and without dasabuvir and with and without ribavirin. Most patients with these severe outcomes had evidence of advanced or decompensated cirrhosis prior to initiating therapy. Although causality is difficult to establish due to background advanced liver disease, a potential risk cannot be excluded.
Viekirax is contraindicated in patients with moderate to severe hepatic impairment (Child-Pugh B or C) (see sections 4.2, 4.3, 4.8 and 5.2).
For patients with cirrhosis:
- Monitoring should be performed for clinical signs and symptoms of hepatic decompensation (such as ascites, hepatic encephalopathy, variceal haemorrhage).
- Hepatic laboratory testing including direct bilirubin levels should be performed at baseline, during the first 4 weeks of starting treatment and as clinically indicated thereafter.
- Treatment should be discontinued in patients who develop evidence of hepatic decompensation.
ALT elevations
During clinical trials with Viekirax and dasabuvir with or without ribavirin, transient elevations of ALT to greater than 5 times the upper limit of normal occurred in approximately 1% of subjects (35 of 3,039). ALT elevations were asymptomatic and generally occurred during the first 4 weeks of treatment, without concomitant elevations of bilirubin, and declined within approximately two weeks of onset with continued dosing of Viekirax and dasabuvir with or without ribavirin.
These ALT elevations were significantly more frequent in the subgroup of subjects who were using ethinyloestradiol-containing medicinal products such as combined oral contraceptives or contraceptive vaginal rings (6 of 25 subjects); (see section 4.3). In contrast, the rate of ALT elevations in subjects using other types of oestrogens as typically used in hormonal replacement therapy (i.e., oral and topical oestradiol and conjugated oestrogens) was similar to the rate observed in subjects who were not using oestrogen-containing products (approximately 1% in each group).
Patients who are taking ethinyloestradiol-containing medicinal products (i.e. most combined oral contraceptives or contraceptive vaginal rings) must switch to an alternative method of contraception (e.g., progestin only contraception or non-hormonal methods) prior to initiating Viekirax with or without dasabuvir therapy (see sections 4.3 and 4.5).
Although ALT elevations associated with Viekirax and dasabuvir have been asymptomatic, patients should be instructed to watch for early warning signs of liver inflammation, such as fatigue, weakness, lack of appetite, nausea and vomiting, as well as later signs such as jaundice and discoloured faeces, and to consult a doctor without delay if such symptoms occur. Routine monitoring of liver enzymes is not necessary in patients that do not have cirrhosis (for cirrhotics, see above). Early discontinuation may result in drug resistance, but implications for future therapy are not known.
Pregnancy and concomitant use with ribavirin
Also see section 4.6.
Extreme caution must be taken to avoid pregnancy in female patients and female partners of male patients when Viekirax is taken in combination with ribavirin, see section 4.6 and refer to the Summary of Product Characteristics for ribavirin for additional information.
Use with tacrolimus, sirolimus and everolimus
Co-administration of Viekirax and dasabuvir with systemic tacrolimus, sirolimus or everolimus increases the concentrations of the immunosuppressant due to CYP3A inhibition by ritonavir (see section 4.5). Serious and/or life threatening events have been observed with co-administration of Viekirax and dasabuvir with systemic tacrolimus, and a similar risk can be expected with sirolimus and everolimus.
Avoid concomitant use of tacrolimus or sirolimus with Viekirax and dasabuvir unless the benefits outweigh the risks. If tacrolimus or sirolimus are used together with Viekirax and dasabuvir, caution is advised, and recommended doses and monitoring strategies can be found in section 4.5. Everolimus cannot be used due to lack of suitable dose strengths for dose adjustments.
Tacrolimus or sirolimus whole blood concentrations should be monitored upon initiation and throughout co-administration with Viekirax and dasabuvir and the dose and/or dosing frequency should be adjusted as needed. Patients should be monitored frequently for any changes in renal function or tacrolimus or sirolimus associated adverse reactions. Refer to the tacrolimus or sirolimus Summary of Product Characteristics for additional dosing and monitoring instructions.
Genotype-specific activity
Concerning recommended regimens with different HCV genotypes, see section 4.2. Concerning genotype- specific virological and clinical activity, see section 5.1.
The efficacy of Viekirax has not been established in patients with HCV genotypes 2, 3, 5 and 6; therefore Viekirax should not be used to treat patients infected with these genotypes.
Co-administration with other direct-acting antivirals against HCV
Viekirax safety and efficacy have been established in combination with dasabuvir and/or ribavirin. Coadministration of Viekirax with other antivirals has not been studied and, therefore, cannot be recommended.
Retreatment
The efficacy of Viekirax in patients previously exposed to Viekirax, or to medicinal products of the same classes as those of Viekirax (NS3/4A inhibitors or NS5A inhibitors), has not been demonstrated. Concerning cross-resistance, see also section 5.1.
Use with glucocorticoids metabolised by CYP3A (e.g. fluticasone)
Caution should be used when administering Viekirax with fluticasone or other glucocorticoids that are metabolised by CYP3A4. Concomitant use of inhaled glucocorticoids metabolised with CYP3A can increase systemic exposures of the glucocorticoids, and cases of Cushing’s syndrome and subsequent adrenal suppression have been reported with ritonavir-containing regimens. Concomitant use of Viekirax and glucocorticoids, particularly long-term use, should only be initiated if the potential benefit of treatment outweighs the risk of systemic corticosteroid effects (see section 4.5).
Use with colchicine
The interaction between Viekirax with or without dasabuvir and colchicine has not been evaluated. A reduction in colchicine dosage or an interruption of colchicine treatment is recommended in patients with normal renal or hepatic function if treatment with Viekirax with or without dasabuvir is required (see section 4.5). In patients with renal or hepatic impairment, use of colchicine with Viekirax with or without dasabuvir is contraindicated (see sections 4.3 and 4.5).
Use with statins
Simvastatin, lovastatin and atorvastatin are contraindicated (see sections 4.3 and 4.5).
Rosuvastatin
Viekirax with dasabuvir is expected to increase the exposure to rosuvastatin more than 3-fold. If rosuvastatin treatment is required during the treatment period, the maximum daily dose of rosuvastatin should be 5 mg (see section 4.5, Table 2). The increase in rosuvastatin when combined with Viekirax without dasabuvir is less pronounced. In this combination, the maximum daily dose of rosuvastatin should be 10 mg (see section 4.5, Table 2).
Pitavastatin and fluvastatin
The interactions between pitavastatin and fluvastatin and Viekirax have not been investigated. Theoretically, Viekirax with and without dasabuvir is expected to increase the exposure to pitavastatin and fluvastatin. A temporary suspension of pitavastatin/fluvastatin is recommended for the duration of treatment with Viekirax. If statin treatment is required during the treatment period, a switch to a reduced dose of pravastatin/rosuvastatin is possible (see section 4.5, Table 2).
Treatment of patients with HIV co-infection
Low dose ritonavir, which is part of the fixed dose combination Viekirax, may select for PI resistance in HIV co-infected patients without ongoing antiretroviral therapy. HIV co-infected patients without suppressive antiretroviral therapy should not be treated with Viekirax.
Drug interactions need to be carefully taken into account in the setting of HIV co-infection (for details see section 4.5, Table 2).
Atazanavir can be used in combination with Viekirax and dasabuvir, if administered at the same time. To be noted, atazanavir should be taken without ritonavir, since ritonavir 100 mg once daily is provided as part of Viekirax. The combination carries an increased risk for hyperbilirubinemia (including ocular icterus), in particular when ribavirin is part of the hepatitis C regimen.
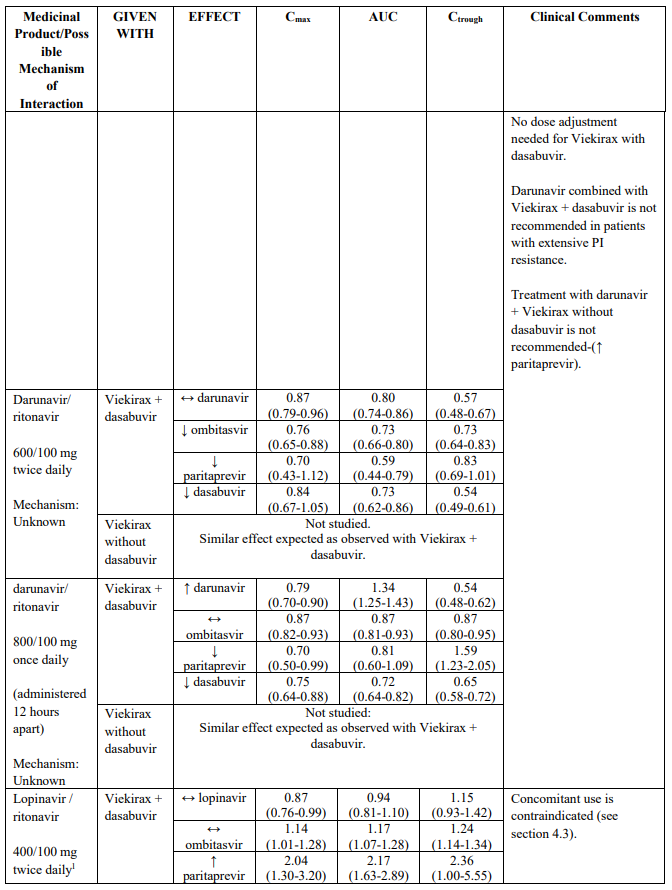
Darunavir, dosed 800 mg once daily, if administered at the same time as Viekirax and dasabuvir, can be used in the absence of extensive PI resistance (darunavir exposure lowered). To be noted, darunavir should be taken without ritonavir, since ritonavir 100 mg once daily is provided as part of Viekirax.
HIV protease inhibitors other than atazanavir and darunavir (e.g., indinavir, saquinavir, tipranavir, lopinavir/ritonavir) are contraindicated (see section 4.3).
Raltegravir exposure is substantially increased (2-fold). The combination was not linked to any particular safety issues in a limited set of patients treated for 12-24 weeks.
Rilpivirine exposure is substantially increased (3-fold) when rilpivirine is given in combination with Viekirax and dasabuvir, with a consequent potential for QT-prolongation. If an HIV protease inhibitor is added (atazanavir, darunavir), rilpivirine exposure may increase even further and is, therefore, not recommended. Rilpivirine should be used cautiously, in the setting of repeated ECG monitoring.
NNRTIs other than rilpivirine (efavirenz, etravirine and nevirapine) are contraindicated (see section 4.3).
Hepatitis B virus reactivation
Cases of hepatitis B virus (HBV) reactivation, some of them fatal, have been reported during or after treatment with direct-acting antiviral medicinal products. HBV screening should be performed in all patients before initiation of treatment. HBV/HCV co-infected patients are at risk of HBV reactivation, and should, therefore, be monitored and managed according to current clinical guidelines.
Depression or psychiatric illness
Cases of depression and more rarely of suicidal ideation and suicide attempt have been reported with Viekirax with or without dasabuvir treatment in combination with ribavirin in the majority of the cases. Although some cases had previous history of depression, psychiatric illness and/or substance abuse, a causal relation with Viekirax with or without dasabuvir treatment cannot be excluded. Caution should be used in patients with a pre-existing history of depression or psychiatric illness. Patients and caregivers should be instructed to notify the prescriber of any changes in behaviour or mood and of any suicidal ideation.
Use in diabetic patients
Diabetics may experience improved glucose control, potentially resulting in symptomatic hypoglycaemia, after initiating HCV direct acting antiviral treatment. Glucose levels of diabetic patients initiating direct acting antiviral therapy should be closely monitored, particularly within the first 3 months, and their diabetic medicinal products modified when necessary. The physician in charge of the diabetic care of the patient should be informed when direct acting antiviral therapy is initiated.
4.5. Interaction with other medicinal products and other forms of interaction
Viekirax may be administered with or without dasabuvir. When co-administered, they exert mutual effects on each other (see section 5.2). Therefore, the interaction profile of the compounds must be considered as a combination.
Pharmacodynamic interactions
Coadministration with enzyme inducers may increase the risk of adverse reactions and ALT eh3.levations (see Table 2). Coadministration with ethinyloestradiol may increase the risk of ALT elevations (see sections 4.3 and 4.4). Examples of contraindicated enzyme inducers are provided in section 4.3.
Pharmacokinetic interactions
Potential for Viekirax to affect the pharmacokinetics of other medicinal products
In vivo drug interaction studies evaluated the net effect of the combination treatment, including ritonavir.
The following section describes the specific transporters and metabolizing enzymes that are affected by Viekirax with or without dasabuvir. See Table 2 for guidance regarding potential interactions with other medicinal products and dosing recommendations.
Medicinal products metabolised by CYP3A4
Ritonavir is a strong inhibitor of CYP3A. Co-administration of Viekirax with or without dasabuvir with medicinal products primarily metabolized by CYP3A may result in increased plasma concentrations of these medicinal products. Medicinal products that are highly dependent on CYP3A for clearance and for which elevated plasma levels are associated with serious events are contraindicated (see section 4.3 and Table 2).
CYP3A substrates evaluated in drug interaction studies which may require dose adjustment and/or clinical monitoring include (see Table 2) ciclosporin, sirolimus, tacrolimus, amlodipine, rilpivirine and alprazolam. Examples of other CYP3A4 substrates which may require dose adjustment and/or clinical monitoring include calcium channel blockers (e.g. nifedipine), and trazodone. Although buprenorphine and zolpidem are also metabolized by CYP3A, drug interaction studies indicate that no dose adjustment is needed when co-administering these medicinal products with Viekirax with or without dasabuvir (see Table 2).
Medicinal products transported by the OATP family and OCT1
Paritaprevir is an inhibitor of the hepatic uptake transporters OATP1B1 and OATP1B3, and paritaprevir and ritonavir are inhibitors of OATP2B1. Ritonavir is an in vitro inhibitor of OCT1, but the clinical relevance is unknown. Co-administration of Viekirax with or without dasabuvir with medicinal products that are substrates of OATP1B1, OATP1B3, OATP2B1 or OCT1 may increase plasma concentrations of these transporter substrates, potentially requiring dose adjustment/clinical monitoring. Such medicinal products include some statins (see Table 2), fexofenadine, repaglinide and angiotensin II receptor antagonists (e.g., valsartan).
OATP1B1/3 substrates evaluated in drug interaction studies include pravastatin and rosuvastatin (see Table 2).
Medicinal products transported by BCRP
Paritaprevir, ritonavir and dasabuvir are inhibitors of BCRP in vivo. Co-administration of Viekirax with or without dasabuvir together with medicinal products that are substrates of BCRP may increase plasma concentrations of these transporter substrates, potentially requiring dose adjustment/clinical monitoring. Such medicinal products include sulfasalazine, imatinib and some of the statins (see Table 2).
BCRP substrates evaluated in drug interaction studies include rosuvastatin (see Table 2).
Medicinal products transported by P-gp in the intestine
While paritaprevir, ritonavir and dasabuvir are in vitro inhibitors of P-gp, no significant change was observed in the exposure of the P-gp substrate digoxin when administered with Viekirax and dasabuvir. However, co-administration of digoxin with Viekirax without dasabuvir may result in increased plasma concentrations (see Table 2). Viekirax may increase the plasma exposure to medicinal products that are sensitive for changed intestinal P-gp activity (such as dabigatran etexilate).
Medicinal products metabolised by glucuronidation (UGT1A1)
Paritaprevir, ombitasvir and dasabuvir are inhibitors of UGT1A1. Co-administration of Viekirax with or without dasabuvir with medicinal products that are primarily metabolized by UGT1A1 result in increased plasma concentrations of such medicinal products; routine clinical monitoring is recommended for narrow therapeutic index medicinal products (i.e. levothyroxine). See also Table 2 for specific advice on raltegravir and buprenorphine, which have been evaluated in drug interaction studies.
Medicinal products metabolised by CYP2C19
Co-administration of Viekirax with or without dasabuvir can decrease exposures of medicinal products that are metabolized by CYP2C19 (e.g. lansoprazole, esomeprazole, s-mephenytoin), which may require dose adjustment/clinical monitoring. CYP2C19 substrates evaluated in drug interaction studies include omeprazole and escitalopram (see Table 2).
Medicinal products metabolised by CYP2C9
Viekirax administered with or without dasabuvir did not affect the exposures of the CYP2C9 substrate, warfarin. Other CYP2C9 substrates (NSAIDs (e.g. ibuprofen), antidiabetics (e.g. glimepiride, glipizide) are not expected to require dose adjustments.
Medicinal products metabolised by CYP2D6 or CYP1A2
Viekirax administered with or without dasabuvir did not affect the exposures of the CYP2D6/CYP1A2 substrate, duloxetine. Exposures of cyclobenzaprine, a CYP1A2 substrate, were decreased. Clinical monitoring and dose adjustment may be needed for other CYP1A2 substrates (e.g. ciprofloxacin, cyclobenzaprine, theophylline and caffeine). CYP2D6 substrates (e.g. desipramine, metoprolol and dextromethorphan) are not expected to require dose adjustments.
Medicinal products renally excreted via transport proteins
Ombitasvir, paritaprevir, and ritonavir do not inhibit organic anion transporter (OAT1) in vivo as shown by the lack of interaction with tenofovir (OAT1 substrate). In vitro studies show that ombitasvir, paritaprevir, and ritonavir are not inhibitors of organic cation transporters (OCT2), organic anion transporters (OAT3), or multidrug and toxin extrusion proteins (MATE1 and MATE2K) at clinically relevant concentrations.
Therefore, Viekirax with or without dasabuvir is not expected to affect medicinal products which are primarily excreted by the renal route via these transporters (see section 5.2).
Potential for other medicinal products to affect the pharmacokinetics of ombitasvir, paritaprevir, and dasabuvir
Medicinal products that inhibit CYP3A4
Co-administration of Viekirax with or without dasabuvir with strong inhibitors of CYP3A may increase paritaprevir concentrations (see section 4.3 and Table 2).
Enzyme inducers
Co-administration of Viekirax and dasabuvir with medicinal products that are moderate or strong enzyme inducers is expected to decrease ombitasvir, paritaprevir, ritonavir and dasabuvir plasma concentrations and reduce their therapeutic effect. Contraindicated enzyme inducers are provided in section 4.3 and Table 2.
Medicinal products that inhibit CYP3A4 and transport proteins
Paritaprevir is eliminated via CYP3A4 mediated metabolism and biliary excretion (substrate of the hepatic transporters OATP1B1, P-gp and BCRP). Caution is advised if co-administering Viekirax with medicinal products that are both moderate inhibitors of CYP3A4 and inhibitors of multiple transporters (P-gp, BCRP and/or OATP1B1/ OATP1B3). These medicinal products may show clinically relevant increases in exposures of paritaprevir (e.g., ritonavir with atazanavir, erythromycin, diltiazem or verapamil).
Medicinal products that inhibit transport proteins
Potent inhibitors of P-gp, BCRP, OATP1B1 and/or OATP1B3 have the potential to increase the exposure to paritaprevir. Inhibition of these transporters is not expected to show clinically relevant increases in exposures of ombitasvir and dasabuvir.
Patients treated with vitamin K antagonists
As liver function may change during treatment with Viekirax administered with or without dasabuvir, a close monitoring of International Normalised Ratio (INR) values is recommended.
Drug interaction studies
Recommendations for co-administration of Viekirax with and without dasabuvir for a number of medicinal products are provided in Table 2.
If a patient is already taking medicinal product(s) or initiating a medicinal product while receiving Viekirax with or without dasabuvir for which potential for drug interaction is expected, dose adjustment of the concomitant medicinal product(s) or appropriate clinical monitoring should be considered (Table 2).
If dose adjustments of concomitant medicinal products are made due to treatment with Viekirax or Viekirax with dasabuvir, doses should be re-adjusted after administration of Viekirax or Viekirax with dasabuvir is completed.
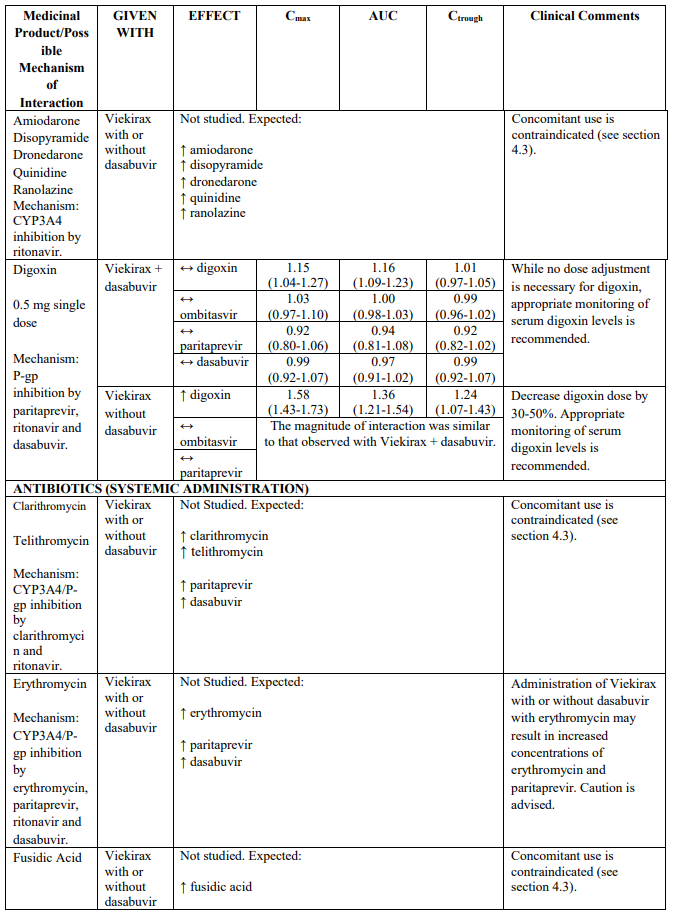
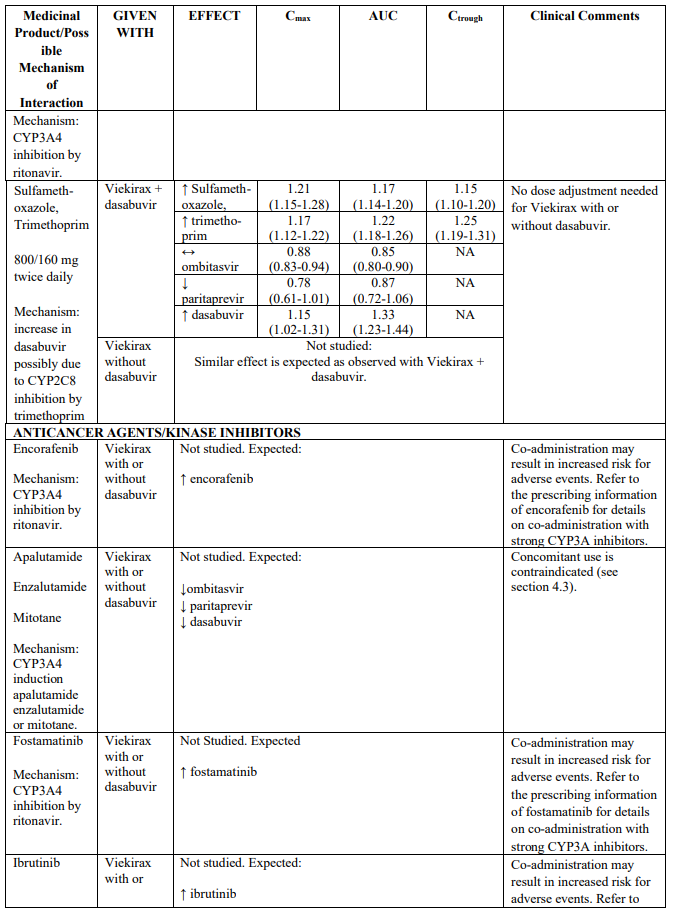
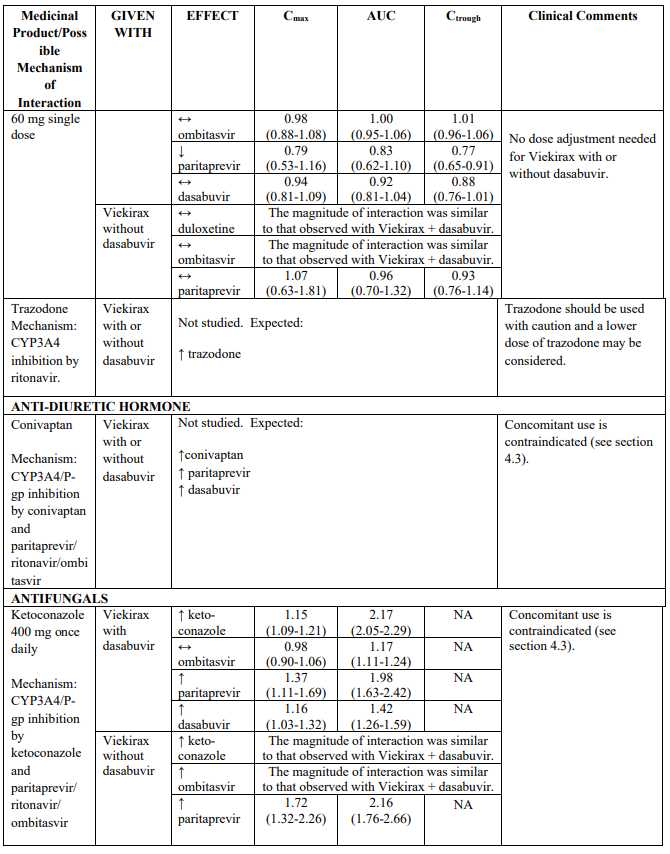
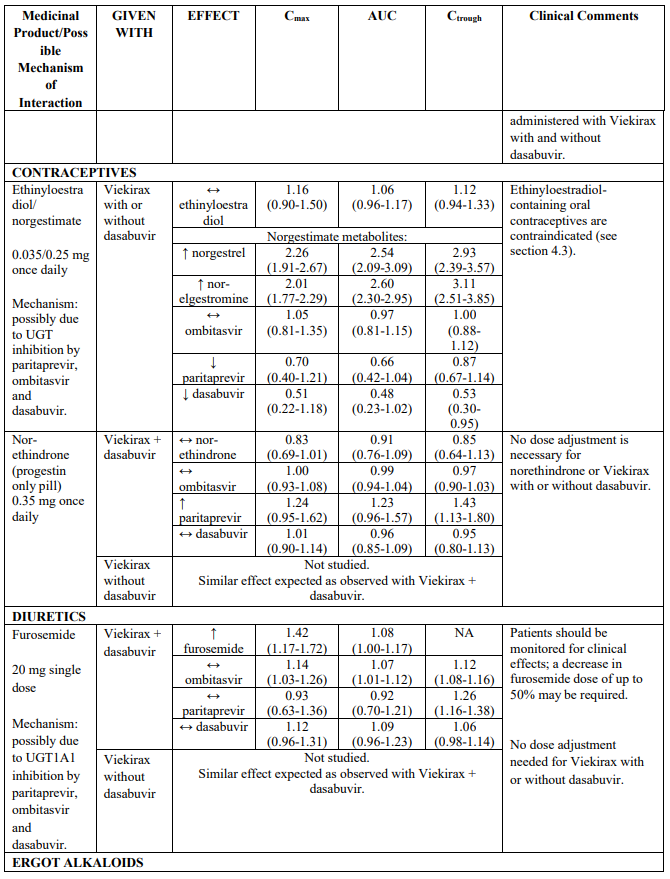
Table 2 provides the Least Squares Means Ratio (90% Confidence Interval) effect on concentration of Viekirax with or without dasabuvir and concomitant medicinal products.
The magnitude of interaction when administered with medicinal products listed in Table 2 are similar (≤25% difference in the Least Square Means ratio) for Viekirax with or without dasabuvir, unless otherwise noted. Drug interactions were evaluated for the Viekirax and dasabuvir regimen, but not for the Viekirax without dasabuvir, with carbamazepine, furosemide, zolpidem, darunavir twice daily, darunavir (evening administration), atazanavir (evening administration), rilpivirine, abacavir/lamivudine, dolutegravir, metformin, sulfamethoxazole/trimethoprim, cyclobenzaprine, carisoprodol, hydrocodone/ paracetamol or diazepam. Thus, for these medicinal products, results and dosing recommendations of the Viekirax and dasabuvir regimen can be extrapolated to Viekirax without dasabuvir.
The direction of the arrow indicates the direction of the change in exposures (Cmax, and AUC) in paritaprevir, ombitasvir, dasabuvir and the co-administered medicinal product (↑ = increase (more than 20%), ↓ = decrease (of more than 20%), ↔ = no change or change less than 20%). This is not an exclusive list.
Table 2. Interactions between Viekirax with or without dasabuvir and other medicinal products:
1 Lopinavir/ritonavir 800/200 mg once daily (administered in the evening) was also administered with Viekirax with or without dasabuvir. The effect on Cmax and AUC of DAAs and lopinavir was similar to that observed when lopinavir/ritonavir 400/100 mg twice daily was administered with Viekirax with or without dasabuvir.
2 Rilpivirine was also administered in the evening with food and at night 4 hours after dinner with Viekirax + dasabuvir in other two arms in the study. The effect on rilpivirine exposures was similar to that observed when rilpivirine was administered in the morning with food with Viekirax + dasabuvir (shown in the table above).
3 Ciclosporin 100 mg dosed alone, 10 mg administered with Viekirax and 30 mg administered with Viekirax + dasabuvir. Dose normalized cyclosporine ratios are shown for interaction with Viekirax with or without dasabuvir.
4 C12:= concentration at 12 hours following single dose of everolimus.
5 Sirolimus 2 mg was dosed alone, 0.5 mg administered with Viekirax + dasabuvir. Dose normalized sirolimus ratios are shown for interaction with Viekirax + dasabuvir.
6 C24:= concentration at 24 hours following single dose of cyclosporine, tacrolimus or sirolimus.
7 Tacrolimus 2 mg was dosed alone, 0.5 mg administered with Viekirax and 2 mg was administered with Viekirax + dasabuvir. Dose normalized tacrolimus ratios are shown for interaction with Viekirax with or without dasabuvir.
8 Dose normalised parameters reported for methadone, buprenorphine and naloxone.
Note: Doses used for Viekirax and dasabuvir were: ombitasvir 25 mg, paritaprevir 150 mg, ritonavir 100 mg, once daily and dasabuvir 400 mg twice daily or 250 mg twice daily. The dasabuvir exposures obtained with the 400 mg formulation and the 250 mg tablet are similar. Viekirax with or without dasabuvir was administered as multiple doses in all the drug interaction studies except the drug interaction studies with carbamazepine, gemfibrozil, ketoconazole, and sulfamethoxazole/trimethoprim..
Paediatric population
Drug interaction studies have only been performed in adults.
4.6. Pregnancy and lactation
Women of childbearing potential/contraception in males and females
Extreme caution must be taken to avoid pregnancy in female patients and female partners of male patients when Viekirax is taken in combination with ribavirin. Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin; therefore, ribavirin is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Refer to the Summary of Product Characteristics for ribavirin for additional information.
Female patients
Women of childbearing potential should not receive ribavirin unless they are using an effective form of contraception during treatment with ribavirin and for 4 months after treatment. Ethinyloestradiol is contraindicated in combination with Viekirax (see sections 4.3 and 4.4).
Male patients and their female partners
Either male patients or their female partners of childbearing potential must use a form of effective contraception during treatment with ribavirin and for 7 months after treatment.
Pregnancy
There are very limited data from the use of Viekirax in pregnant women. Studies with ombitasvir and paritaprevir/ritonavir in animals have shown malformations (see section 5.3). The potential risk for humans is unknown. Viekirax should not be used during pregnancy or in women of childbearing potential not using effective contraception.
If ribavirin is co-administered with Viekirax, the contraindications regarding use of ribavirin during pregnancy apply (see also the Summary of Product Characteristics of ribavirin).
Breast-feeding
It is not known whether paritaprevir/ritonavir or ombitasvir and their metabolites are excreted in human breast milk. Available pharmacokinetic data in animals have shown excretion of active substance and metabolite in milk (see section 5.3). Because of the potential for adverse reactions from the medicinal product in breastfed infants, a decision must be made whether to discontinue breast-feeding or discontinue treatment with Viekirax, taking into account the importance of the therapy to the mother. For patients coadministered ribavirin refer to the Summary of Product Characteristics of ribavirin.
Fertility
No human data on the effect of Viekirax on fertility are available. Animal studies do not indicate harmful effects on fertility (see section 5.3).
4.7. Effects on ability to drive and use machines
Viekirax has no or negligible influence on the ability to drive and use machines. Patients should be informed that fatigue has been reported during treatment with Viekirax in combination with dasabuvir and ribavirin (see section 4.8).
4.8. Undesirable effects
Summary of the safety profile
In subjects receiving Viekirax and dasabuvir with ribavirin, the most commonly reported adverse reactions (greater than 20% of subjects) were fatigue and nausea. The proportion of subjects who permanently discontinued treatment due to adverse reactions was 0.2% (5/2,044) and 4.8% (99/2,044) of subjects had ribavirin dose reductions due to adverse reactions.
Tabulated list of adverse reactions
The safety summary is based on pooled data from phase 2 and 3 clinical trials in subjects who received Viekirax and dasabuvir with or without ribavirin. The majority of adverse reactions presented in Table 3 were of grade 1 severity in Viekirax and dasabuvir-containing regimens.
The adverse reactions are listed below by system organ class and frequency. Frequencies are defined as follows: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1,000 to <1/100), rare (≥1/10,000 to <1/1,000) or very rare (<1/10,000).
Table 3. Adverse drug reactions identified with Viekirax in combination with dasabuvir with and without ribavirin:
| Frequency | Viekirax + dasabuvir + ribavirin* N=2,044 | Viekirax + dasabuvir N=588 |
|---|---|---|
| Blood and lymphatic system disorders | ||
| Common | Anaemia | |
| Immune system disorders | ||
| Frequency unknown | Anaphylactic reactions | Anaphylactic reactions |
| Metabolism and nutrition disorders | ||
| Uncommon | Dehydration | |
| Psychiatric disorders | ||
| Very common | Insomnia | |
| Gastrointestinal disorders | ||
| Very common | Nausea, Diarrhoea | |
| Common | Vomiting | |
| Hepatobiliary disorders | ||
| Frequency unknown | Hepatic decompensation and hepatic failure | Hepatic decompensation and hepatic failure |
| Skin and subcutaneous tissue disorders | ||
| Very common | Pruritus | |
| Common | Pruritus | |
| Rare | Angioedema | Angioedema |
| General disorders and administration and administration site conditions | ||
| Very common | Asthenia Fatigue | |
* Data set includes all genotype 1-infected subjects in Phase 2 and 3 trials including subjects with cirrhosis.
Note: For laboratory abnormalities, refer to Table 4
Description of selected adverse reactions
Compared to subjects without cirrhosis, in subjects with compensated cirrhosis there was an increased rate of indirect hyperbilirubinemia when ribavirin was part of the regimen.
Laboratory abnormalities
Changes in selected laboratory parameters are described in Table 4. A side-by-side tabulation is shown to simplify presentation; direct comparison across trials should not be made due to differing trial designs.
Table 4. Selected treatment emergent laboratory abnormalities:
| Laboratory Parameters | SAPPHIRE I and II | PEARL II, III, and IV | TURQUOISE II (subjects with cirrhosis) |
|---|---|---|---|
| Viekirax and dasabuvirv+ ribavirin 12 weeks N=770 n (%) | Viekirax and dasabuvir 12 weeks N=509 n (%) | Viekirax and dasabuvir + ribavirin 12 or 24 weeks N=380 n (%) | |
| ALT | |||
| >5-20 × ULN* (Grade 3) | 6/765 (0.8%) | 1/509 (0.2%) | 4/380 (1.1%) |
| >20 × ULN (Grade 4) | 3/765 (0.4%) | 0 | 2/380 (0.5%) |
| Haemoglobin | |||
| <100-80 g/L (grade 2) | 41/765 (5.4%) | 0 | 30/380 (7.9%) |
| <80-65 g/L (grade 3) | 1/765 (0.1%) | 0 | 3/380 (0.8%) |
| <65 g/L (Grade 4) | 0 | 0 | 1/380 (0.3%) |
| Total bilirubin | |||
| >3-10 × ULN (grade 3) | 19/765 (2.5%) | 2/509 (0.4%) | 37/380 (9.7%) |
| >10 × ULN (grade 4) | 1/765 (0.1%) | 0 | 0 |
* ULN: Upper limit of normal according to testing laboratory.
Serum ALT elevations
In a pooled analysis of clinical trials with Viekirax and dasabuvir with and without ribavirin, 1% of subjects experienced serum ALT levels greater than 5 times the upper limit of normal (ULN) after starting treatment. As the incidence of such elevations was 26% among women taking a concomitant ethinyloestradiol-containing medicinal product, such medicinal products are contraindicated with Viekirax with or without dasabuvir. No increase in incidence of ALT elevations was observed with other types of estrogens commonly used for hormone replacement therapy (e.g. oestradiol and conjugated estrogens). ALT elevations were typically asymptomatic, generally occurred during the first 4 weeks of treatment (mean time 20 days, range 8-57 days) and most resolved with ongoing therapy. Two patients discontinued Viekirax and dasabuvir due to elevated ALT, including one on ethinyloestradiol. Three interrupted Viekirax and dasabuvir for one to seven days, including one on ethinyloestradiol. The majority of these ALT elevations were transient and assessed as drug-related. Elevations in ALT were generally not associated with bilirubin elevations. Cirrhosis was not a risk factor for elevated ALT (see section 4.4).
Serum bilirubin elevations
Transient elevations in serum bilirubin (predominantly indirect) were observed in subjects receiving Viekirax and dasabuvir with ribavirin, related to the inhibition of the bilirubin transporters OATP1B1/1B3 by paritaprevir and ribavirin-induced haemolysis. Bilirubin elevations occurred after initiation of treatment, peaked by study Week 1, and generally resolved with ongoing therapy. Bilirubin elevations were not associated with aminotransferase elevations. The frequency of indirect bilirubin elevations was lower among subjects who did not receive ribavirin.
Liver transplant recipients
The overall safety profile in HCV-infected transplant recipients who were administered Viekirax and dasabuvir and ribavirin (in addition to their immunosuppressant medications) was similar to subjects treated with Viekirax and dasabuvir and ribavirin in phase 3 clinical trials, although some adverse reactions were increased in frequency. 10 subjects (29.4%) had at least one post baseline haemoglobin value of less than 10 g/dL. 10 of 34 subjects (29.4%) dose modified ribavirin due to decrease in haemoglobin and 2.9% (1/34) had an interruption of ribavirin. Ribavirin dose modification did not impact SVR rates. 5 subjects required erythropoietin, all of whom initiated ribavirin at the starting dose of 1000 to 1200 mg daily. No subject received a blood transfusion.
HIV/HCV co-infected patients
The overall safety profile in HCV/HIV-1 co-infected subjects was similar to that observed in HCV monoinfected subjects. Transient elevations in total bilirubin >3 x ULN (mostly indirect) occurred in 17 (27.0%) subjects; 15 of these subjects were receiving atazanavir. None of the subjects with hyperbilirubinemia had concomitant elevations of aminotransferases.
GT1-infected subjects with or without cirrhosis with severe renal impairment or end-stage renal disease (ESRD)
Viekirax and dasabuvir with or without ribavirin were assessed in 68 subjects with genotype 1 infection with or without cirrhosis who have severe renal impairment or ESRD (see Section 5.1). The overall safety profile in subjects with severe renal impairment was similar to that seen in prior Phase 3 studies in subjects without severe renal impairment, except that a greater proportion of subjects required intervention due to ribavirin-associated decreases in serum haemoglobin. The mean baseline haemoglobin level was 12.1 g/dL and the mean decline in haemoglobin at the end of treatment for subjects taking RBV was 1.2 g/dL. Thirty-nine of the 50 subjects who received ribavirin required interruption of ribavirin, and 11 of these subjects were also treated with erythropoietin. Four subjects experienced a haemoglobin level <8 g/dL. Two subjects received a blood transfusion. Adverse events of anaemia were not seen in the 18 GT1b-infected subjects who did not receive ribavirin. Viekirax with or without dasabuvir was also evaluated without ribavirin in 18 GT1a- and GT4-infected patients; no adverse events of anaemia were seen in these subjects.
Paediatric population
The safety of Viekirax in children and adolescents aged <18 years has not yet been established. No data are available.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.