VIRAZOLE Inhalation solution Ref.[27429] Active ingredients: Ribavirin
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
VIRAZOLE (Ribavirin for Inhalation Solution, USP) is a brand name for ribavirin, a synthetic nucleoside with antiviral activity. VIRAZOLE for inhalation solution is a sterile, lyophilized powder to be reconstituted for aerosol administration. Each 100 mL glass vial contains 6 grams of ribavirin, and when reconstituted to the recommended volume of 300 mL with Sterile Water for Injection, USP or Sterile Water for Inhalation (no preservatives added), will contain 20 mg of ribavirin per mL, pH approximately 5.5. Aerosolization is to be carried out in a Small Particle Aerosol Generator (SPAG-2) nebulizer only.
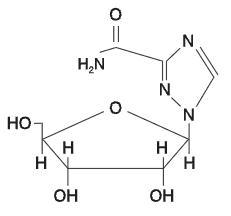
Ribavirin is 1-beta-D-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide, with the following structural formula:
Ribavirin is a stable, white crystalline compound with a maximum solubility in water of 142 mg/mL at 25°C and with only a slight solubility in ethanol. The empirical formula is C8H12N4O5 and the molecular weight is 244.21.
| How Supplied |
|---|
|
VIRAZOLE (Ribavirin for Inhalation Solution, USP) is supplied in one-pack and four-pack counts containing 100 mL glass vials with 6 grams of Sterile, lyophilized drug, which is to be reconstituted with 300 mL Sterile Water for Injection, USP or Sterile Water for Inhalation (no preservatives added) and administered only by a small particle aerosol generator (SPAG-2). NDC 0187-0007-01 One 6 g glass vial Manufactured for: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Hospira, Inc., McPherson, KS 67460 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| VIRAZOLE | Canada, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.