VIZZ Ophthalmic solution Ref.[115527] Active ingredients: Aceclidine
Source: FDA, National Drug Code (US) Revision Year: 2025
12.1. Mechanism of Action
Aceclidine is a cholinergic muscarinic agonist that stimulates muscarinic receptors located on smooth muscles. VIZZ is a predominately pupil selective miotic that interacts with the iris with minimal ciliary muscle stimulation. VIZZ causes contraction of the iris sphincter muscle, resulting in a pinhole effect that extends depth of focus to improve vision.
12.3. Pharmacokinetics
Aceclidine undergoes hydrolysis in the eye to acetate and 3-quinuclidinol (3-Q) with one mole of aceclidine hydrolyzed to one mole of 3-Q. Pharmacokinetic studies are performed on the analysis of 3-Q, which is a metabolite formed from the hydrolysis of aceclidine.
Systemic exposure of aceclidine hydrochloride was evaluated in 16 subjects with presbyopia following once daily VIZZ administration (one drop of VIZZ in each eye followed by a second drop in each eye two minutes later) for 8 days. The mean Cmax and AUC0-t values for 3-Q after Day 8 dosing were 2.114 ng/mL and 4.899 hr*ng/mL, respectively.
There was little to no accumulation of 3-Q after repeat once daily dosing of VIZZ. After 8 days, the mean (SD) RAUC0-t and RCmax values were 1.189 (0.770) and 0.996 (0.314), respectively. T1/2 could not be estimated at any timepoint due to the limited amount of quantifiable 3-Q concentrations after ocular dosing.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long term studies in animals have not been performed to evaluate carcinogenic potential.
Mutagenesis
Aceclidine did not show any potential to cause genetic toxicity in a series of studies that included: 1) bacterial assays (Salmonella and E. coli) for reverse gene mutations; 2) an in vitro chromosome aberration assay in cultured human peripheral blood lymphocytes; and 3) an in vivo chromosome aberration assay (micronucleus test) in mice.
Impairment of Fertility
Oral administration of aceclidine produced no adverse effect on fertility in rats at doses up to 1.5 mg/kg/day (approximately 25 times the MRHOD based on body surface area).
14. Clinical Studies
The efficacy of VIZZ for the treatment of presbyopia was demonstrated in two, randomized, double-masked, controlled studies, CLARITY-1 and CLARITY-2. A total of 466 participants aged 45 to 75 years old with presbyopia were randomized. Participants had a refractive range from -4.00 to +1.00 D sphere, with astigmatism up to 2.00 D, and a spherical equivalent no more myopic than -4.00 D. Both studies included participants who were post-refractive surgery and/or pseudophakic.
Participants were instructed to instill 2 drops of VIZZ (or control) in each eye once daily, one drop in each eye followed by a second drop in each eye two minutes later. Participants were treated for 42 days. Ophthalmic efficacy assessments were conducted on Day 1, Day 15, and Day 28 of the study at various timepoints through 10 hours post dose.
In each study, the proportion of participants gaining 3 lines or more in high contrast, distance corrected, near visual acuity (DCNVA) at 40 cm, without loss of 1 line or more (≥5 letters) of distance corrected, distance visual acuity (DCDVA) at 4 meters was statistically significantly greater in the VIZZ group compared to the control group at Day 1, Hour 3.
Table 1. Primary Efficacy Endpoint from CLARITY-1 and CLARITY-2 Studies Day 1, at 3 Hours Post Dose (FAS Population):
| CLARITY-1 | CLARITY-2 | |||||
|---|---|---|---|---|---|---|
| VIZZ N=157 | Brimonidine N=156 | p-value | VIZZ N=77 | Vehicle N=76 | p-value | |
| Proportion of participants gaining 3-lines or more in DCNVA at 40cm, without losing 1 line or more (≥5 letters) of DCDVA at 4m at Day 1, at 3 hours | 65% | 12% | p<0.01 | 71% | 8% | p<0.01 |
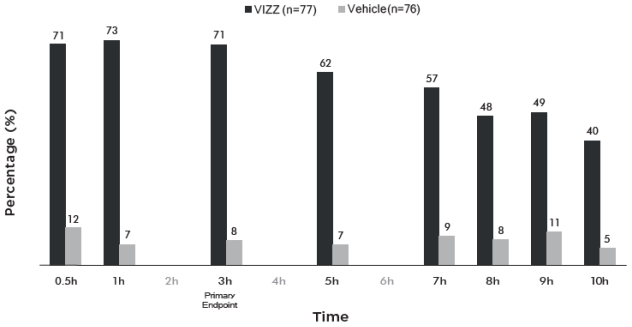
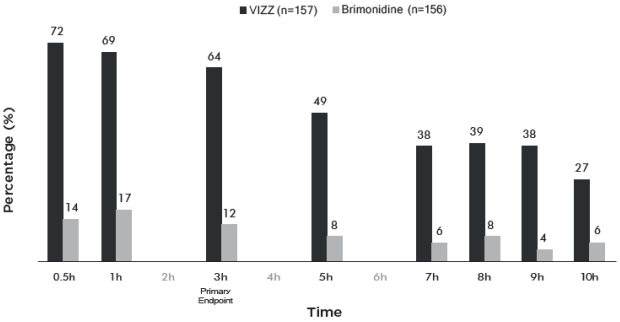
Figure 1 and Figure 2 demonstrate the onset of the VIZZ effect on presbyopia, from 30 minutes post dose and lasting 10 hours.
Figure 1. Percentage of Participants Achieving 3 Lines or More Improvement in High Contrast, Monocular Near Vision (DCNVA at 40 cm) and No Loss of 1 or More Lines (DCDVA at 4 m) on Day 1 at All Measured Time Points (CLARITY-2, FAS population with Observed data):
Figure 2. Percentage of Participants Achieving 3 Lines or More Improvement in High Contrast, Monocular Near Vision (DCNVA at 40 cm) and No Loss of 1 or More Lines (DCDVA at 4 m) on Day 1 at All Measured Time Points (CLARITY-1, FAS population with Observed data):
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.