VOLUTRIP Film-coated tablet Ref.[50539] Active ingredients: Dolutegravir Lamivudine Tenofovir disoproxil
Source: Health Products Regulatory Authority (ZA) Revision Year: 2018 Publisher: Aurobindo Pharma (Pty) Ltd, Woodhill Office Park, Building 1, 53 Phillip Engelbrecht Avenue, Meyersdal, Ext. 12, 1448, Johannesburg, South Africa
4.3. Contraindications
VOLUTRIP tablets are contra-indicated in patients with known hypersensitivity to lamivudine, tenofovir or dolutegravir or to any of the components of the tablets.
Impairment of renal function.
Pregnancy and lactation (see Pregnancy and lactation).
Women of child-bearing age not using highly effective contraception.
Concomitant use with adefovir dipivoxil.
Co-administration with dofetilide and pilsicainide.
Co-administration with didanosine.
Co-administration with metformin.
Patients younger than 18 years of age.
Moderate and severe hepatic impairment.
4.4. Special warnings and precautions for use
LACTIC ACIDOSIS AND SEVERE HEPATOMEGALY WITH STEATOSIS, INCLUDING FATAL CASES, HAVE BEEN REPORTED WITH THE USE OF NUCLEOSIDE ANALOGUES ALONE OR IN COMBINATION WITH OTHER ANTIRETROVIRALS (SEE WARNINGS AND SPECIAL PRECAUTIONS).
VOLUTRIP IS NOT INDICATED FOR THE TREATMENT OF CHRONIC HEPATITIS B VIRUS (HBV) INFECTION. THE SAFETY AND EFFICACY OF VOLUTRIP HAS NOT BEEN ESTABLISHED IN PATIENTS CO-INFECTED WITH HBV AND HIV. SEVERE ACUTE EXACERBATIONS OF HEPATITIS B HAVE BEEN REPORTED IN PATIENTS WHO ARE COINFECTED WITH HBV AND HIV AND HAVE DISCONTINUED THE COMBINATION TABLET.
HEPATIC FUNCTION SHOULD BE MONITORED CLOSELY WITH BOTH CLINICAL AND LABORATORY FOLLOW-UP FOR AT LEAST SEVERAL MONTHS IN PATIENTS WHO DISCONTINUE VOLUTRIP AND ARE CO-INFECTED WITH HIV AND HBV. IF APPROPRIATE, INITIATION OF ANTI-HEPATITIS B THERAPY MAY BE WARRANTED (SEE WARNINGS AND SPECIAL PRECAUTIONS).
Safety and efficacy of the individual active ingredients in various antiretroviral combination regimens with similar dosages as contained in VOLUTRIP have been established in clinical studies for the treatment of HIV patients. However, safety and efficacy of the fixed-drug combination as in VOLUTRIP for the teatment of HIV has not been established in clinical studies. The complete professional informations of the other medicines used in combination should be consulted before initiation of therapy.
Metabolic abnormalities
Combination antiretroviral therapy, including VOLUTRIP has been associated with metabolic abnormalities such as hypertriglyceridaemia, hypercholesterolaemia, insulin resistance, hyperglycaemia and hyperlactataemia.
Lipodystrophy
Combination antiretroviral therapy, including VOLUTRIP, has also been associated with the redistribution/accumulation of body fat, including central obesity, dorso-cervical fat enlargement (buffalo hump), peripheral wasting, facial wasting and breast enlargement in HIV patients.
A higher risk of lipodystrophy has been associated with individual factors such as older age, and with medicine related factors such as longer duration of antiretroviral treatment and associated metabolic disturbances. Clinical examination should include evaluation for physical signs of fat redistribution. Fasting serum lipids and blood glucose levels should be monitored. Lipid disorders should be managed as clinically appropriate. Patients with evidence of lipodystrophy should also have a thorough cardiovascular risk assessment.
Immune Reconstitution Inflammatory Syndrome
Immune Reconstitution Inflammatory Syndrome (IRIS) is an immunopathological response resulting from the rapid restoration of pathogen-specific immune responses to pre-existing antigens combined with immune dysregulation, which occurs shortly after starting combination Anti-Retroviral Therapy (cART). Typically, such reactions present by paradoxical deterioration of opportunistic infections being treated or with unmasking of an asymptomatic opportunistic disease, often with an atypical inflammatory presentation. IRIS usually develops within the first three months of initiation of ART and occurs more commonly in patients with low CD4 counts. Common examples of IRIS reactions to opportunistic diseases are tuberculosis, atypical mycobacterial infections, cytomegalovirus retinitis, Pneumocystis jirovecii, and cryptococcal meningitis.
Appropriate treatment of the opportunistic disease should be instituted or continued and ART continued. Inflammatory manifestations generally subside after a few weeks. Severe cases may respond to glucocorticoids, but there is only limited evidence for this in patients with tuberculosis IRIS. Autoimmune disorders (such as Graves' disease, Guillain-Barre Syndrome, Polymyositis) have also been reported as IRIS reactions; however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported, particularly in patients with advanced HIV-disease and/or long-term exposure to combination antiretroviral therapy (cART), including components of VOLUTRIP. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Opportunistic infections
Patients receiving VOLUTRIP may continue to develop opportunistic infections and other complications of HIV infection and therefore patients should remain under close clinical observation by doctors experienced in the treatment of patients with HIV associated diseases.
The risk of HIV transmission to others
Patients should be advised that treatment with VOLUTRIP, has not been proven to prevent the risk of transmission of HIV to others through sexual contact or blood contamination. Appropriate precautions should continue to be taken.
Lactic acidosis/severe hepatomegaly with steatosis
Lactic acidosis, usually associated with hepatic steatosis, including fatal cases, has been reported with the use of nucleoside analogues, such as in VOLUTRIP. Early symptoms (symptomatic hyperlactataemia) include benign digestive symptoms (nausea, vomiting and abdominal pain), nonspecific malaise, loss of appetite, weight loss, respiratory symptoms (rapid and/or deep breathing) or neurological symptoms (including motor weakness). Lactic acidosis has a high mortality and may be associated with pancreatitis, liver failure or renal failure.
Lactic acidosis generally occurs after a few or several months of treatment. Treatment with nucleoside analogues should be discontinued in the setting of symptomatic hyperlactataemia and metabolic/lactic acidosis, progressive hepatomegaly, or rapidly elevating aminotransferase levels.
Suspicious biochemical features include mild raised transaminases, raised lactate dehydrogenase (LDH) and/or creatine kinase.
In patients with suspicious symptoms or biochemistry, measure the venous lactate level (normal <2 mmol/litre) and respond as follows:
- Lactate 2-5 mmol/litre: monitor regularly, and be alert for clinical signs.
- Lactate 5-10 mmol/litre without symptoms: monitor closely.
- Lactate 5-10 mmol/litre with symptoms: STOP all therapy. Exclude
- other causes (e.g. sepsis, uraemia, diabetic ketoacidosis, hyperthyroidism, lymphoma).
- Lactate >10 mmol/litre: STOP all therapy (80% mortality in case studies).
The above lactate values may not be applicable to paediatric patients.
Diagnosis of lactic acidosis is confirmed by demonstrating metabolic acidosis with an increased anion gap and raised lactate level. Therapy should be stopped in any acidotic patient with a raised lactate level.
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases have been reported with the use of VOLUTRIP alone or in combination, in the treatment of HIV infection. Most cases were women. Caution should be exercised when administering VOLUTRIP to patients with known risk factors for liver disease.
Treatment with VOLUTRIP should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or hepatoxicity. Caution should be exercised when administering nucleoside analogues as contained in VOLUTRIP to any patient (particularly obese women) with hepatomegaly, hepatitis or other known risk factors for liver disease and hepatic steatosis (including certain medicines and alcohol). Patients co-infected with Hepatitis C and treated with alpha interferon and ribavirin may constitute a special risk. Patients at increased risk should be followed closely. However, cases have also been reported in patients with no known risk factors.
There are no study results demonstrating the effect of VOLUTRIP on clinical progression of HIV-1.
Mitochondrial dysfunction
Nucleoside and nucleotide analogues as contained in VOLUTRIP have been demonstrated in vitro and in vivo to cause a variable degree of mitochondrial damage. There have been reports of mitochondrial dysfunction in HIV negative infants exposed in utero and/or postnatally to nucleoside analogues. The main adverse events reported are hematological disorders (anaemia, neutropenia), metabolic disorders (hyperlactatemia, hyperlipidemia). These events are often transitory. Some lateonset neurological disorders have been reported (hypertonia, convulsion, abnormal behaviour). Whether the neurological disorders are transient or permanent is unknown. Any child exposed in utero to nucleoside and nucleotide analogues, even HIV negative children, should have clinical and laboratory follow-up and should be fully investigated for possible mitochondrial dysfunction in case of relevant signs or symptoms.
Pancreatitis
Pancreatitis has been observed in some patients receiving lamivudine, as in VOLUTRIP. It is unclear whether this is due to lamivudine or to underlying HIV disease. Pancreatitis must be considered whenever a patient develops abdominal pain, nausea, vomiting or elevated biochemical markers. Discontinue use of VOLUTRIP until a diagnosis of pancreatitis is excluded.
Patients with renal impairment
In patients with moderate to severe renal impairment, the terminal half-life of VOLUTRIP is increased due to decreased clearance (see CONTRA-INDICATIONS).
Liver disease
Use of VOLUTRIP can result in hepatomegaly due to non-alcoholic fatty liver disease (hepatic steatosis).
The safety and efficacy of VOLUTRIP has not been established in patients with significant underlying liver disorders. Patients with pre-existing liver dysfunction including chronic active hepatitis, have an increased frequency of liver function abnormalities during combination antiretroviral therapy and should be monitored according to standard practice. If there is evidence of worsening liver disease in such patients, interruption or discontinuation of treatment must be considered.
Renal Impairment
VOLUTRIP is a combination medicine and the dose of the individual components cannot be altered. Since VOLUTRIP is primarily eliminated by the kidneys, co-administration of VOLUTRIP with medicines that reduce renal function or compete for active tubular secretion may increase serum concentrations of VOLUTRIP and/or increase the concentrations of other renally eliminated medicines. Some examples include, but are not limited to adefovir dipivoxil, cidofovir, aciclovir, valaciclovir, ganciclovir and valganciclovir.
VOLUTRIP is not recommended for patients with creatinine clearance <80 mL/min or patients who require haemodialysis. Renal impairment, including cases of acute renal failure and Fanconi syndrome (renal tubular injury with severe hypophosphatemia) has been reported in association with the use of tenofovir disoproxil fumarate in clinical practice. Careful monitoring of renal function (serum creatinine and serum phosphate) is therefore recommended before taking VOLUTRIP.
Renal safety with tenofovir has only been studied to a very limited degree in adult patients with impaired renal function (creatinine clearance <80mL/min).
Renal monitoring
It is recommended that renal function (creatinine clearance and serum phosphate) is assessed in all patients prior to initiating therapy with tenofovir disoproxil fumarate and that it is also monitored every four weeks during the first year of tenofovir disoproxil fumarate therapy, and then every three months. In patients at risk for renal impairment, including patients who have previously experienced renal events while receiving adefovir dipivoxil, consideration should be given to more frequent monitoring of renal function.
Co-administration and risk of renal toxicity
Use of tenofovir disoproxil fumarate should be avoided with concurrent or recent of a nephrotoxic medicine (e.g. aminoglycosides, amphotericin B, foscarnet, ganciclovir, pentamidine, vancomycin, cidofovir, or interleukin-2). If concomitant use of tenofovir disoproxil fumarate and nephrotoxic medicines is unavoidable, renal function should be monitored weekly.
Tenofovir disoproxil fumarate has not been clinically evaluated in patients receiving medicines which are secreted by the same renal pathway, including the transport proteins human organic anion transporter (hOAT) 1 and 3 or MRP 4 (e.g. cidofovir, a known nephrotoxic medicine). These renal transport proteins may be responsible for tubular secretion and in part, renal elimination of tenofovir and cidofovir. Consequently, the pharmacokinetics of these medicines, which are secreted by the same renal pathway including transport proteins hOAT 1 and 3 or MRP 4; might be modified if they are co-administered. Unless clearly necessary, concomitant use of these medicines which are secreted by the same renal pathway is not recommended, but if such use is unavoidable, renal function should be monitored weekly.
VOLUTRIP should be avoided with concurrent or recent use of a nephrotoxic medicine. Patients at risk of, or with a history of, renal dysfunction and patients receiving concomitant nephrotoxic substances should be carefully monitored for changes in serum creatinine and phosphorous.
K65R mutation
VOLUTRIP should be avoided in antiretroviral experienced patients with HIV-1 harbouring the K65R mutation.
Bone mineral density
Decreases in bone mineral density of spine and changes in bone biomarkers from baseline are significantly greater with tenofovir disoproxil fumarate as contained in VOLUTRIP. Decreases in bone mineral density of the hip are significantly greater. Clinically relevant bone fractures are reported. If bone abnormalities are suspected then appropriate consultation should be obtained. Bone monitoring should be considered for HIV infected patients who have a history of pathologic bone fracture or are at risk of osteopenia.
VOLUTRIP may cause a reduction in bone mineral density. The effects of tenofovir disoproxil fumarate-associated changes in bone mineral density on long-term bone health and future fracture risk are currently unknown.
Bone monitoring should be considered for HIV infected patients who have a history of pathologic bone fracture or are at risk for osteopenia. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation may be beneficial for all patients. If bone abnormalities are suspected then appropriate consultation should be obtained. Bone abnormalities (infrequently contributing to fractures) may be associated with proximal renal tubulopathy.
Patients with HIV and Hepatitis B or C virus co-infection
VOLUTRIP is not indicated for the treatment of chronic HBV infection. The safety and efficacy of VOLUTRIP has not been established for the treatment of patients co-infected with HBV and HIV.
Patients with chronic hepatitis B or C and treated with antiretroviral therapy VOLUTRIP are at an increased risk for severe and potentially fatal hepatic adverse reactions. Medical practitioners should refer to current HIV treatment guidelines for the optimal management of HIV infection in patients coinfected with hepatitis B virus (HBV). In case of concomitant antiviral therapy for hepatitis B or C, please refer also to the relevant professional informations for these medicines.
Exacerbations of hepatitis
Flares on treatment
Spontaneous exacerbations in chronic hepatitis B are relatively common and are characterised by transient increases in serum ALT. After initiating antiviral therapy, serum ALT may increase in some patients. In patients with compensated liver disease, these increases in serum ALT are generally not accompanied by an increase in serum bilirubin concentrations or hepatic decompensation. Patients with cirrhosis may be at a higher risk for hepatic decompensation following hepatitis exacerbation, and therefore should be monitored closely during therapy.
Flares after treatment discontinuation
Acute exacerbations of hepatitis have been reported in patients after the discontinuation of hepatitis B therapy. Post-treatment exacerbations are usually associated with rising HBV DNA, and the majority appears to be self-limited. However, severe exacerbations, including fatalities, have been reported. Hepatic function should be monitored at repeated intervals with both clinical and laboratory follow-up for at least 6 months after discontinuation of hepatitis B therapy. If appropriate, resumption of hepatitis B therapy may be warranted. In patients with advanced liver disease or cirrhosis, treatment discontinuation is not recommended since post-treatment exacerbations of hepatitis may lead to hepatic decompensation. Liver flares are especially serious, and sometimes fatal in patients with decompensated liver disease.
Hypersensitivity reactions
Hypersensitivity reactions have been reported with integrase inhibitors, including dolutegravir as in VOLUTRIP and were characterised by rash, constitutional findings and sometimes, organ dysfunction, including liver injury. Discontinue VOLUTRIP and other suspect medicines immediately if signs or symptoms of hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial oedema, hepatitis, eosinophilia, angioedema). Clinical status including liver aminotransferases should be monitored and appropriate therapy initiated. Delay in stopping treatment with VOLUTRIP or other suspect medicines after the onset of hypersensitivity may result in a lifethreatening reaction.
Interactions
Caution should be given to co-administering medications (prescription and non-prescription) that may change the exposure of dolutegravir or medications that may have their exposure changed by dolutegravir (see CONTRA-INDICATIONS and INTERACTION").
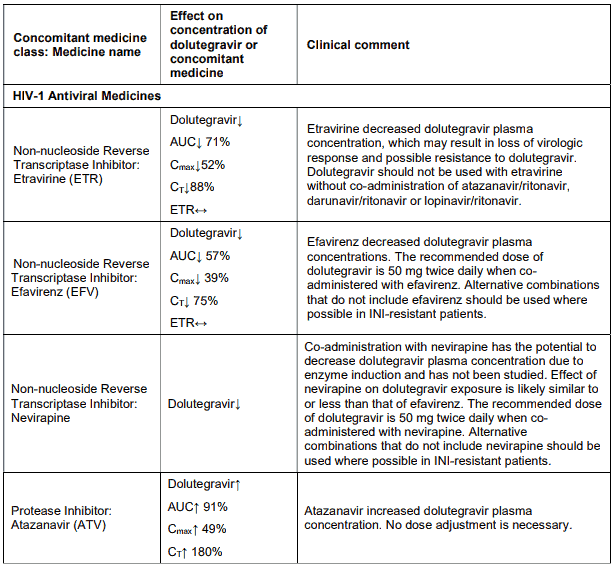
The co-administration of dolutegravir with etravirine (ETR) is not recommended unless the patient is also receiving concomitant atazanavir + ritonavir (ATV + RTV), lopinavir + ritonavir (LPV + RTV) or darunavir + ritonavir (DRV + RTV) (see INTERACTIONS).
The recommended dose of dolutegravir is 50 mg twice daily when co-administered with efavirenz, nevirapine, tipranavir/ritonavir, or rifampicin (see INTERACTIONS).
Dolutegravir should not be co-administered with polyvalent cation-containing antacids. Dolutegravir is recommended to be administered 2 hours before or 6 hours after these medicines (see INTERACTIONS).
Metformin concentrations may be increased by dolutegravir. Metformin is contra-indicated in patients taking dolutegravir (see CONTRA-INDICATIONS).
Paediatric use
Safety and effectiveness in paediatric patients and patients <18 years of age have not been established.
Elderly use
Clinical studies did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients.
4.5. Interaction with other medicinal products and other forms of interaction
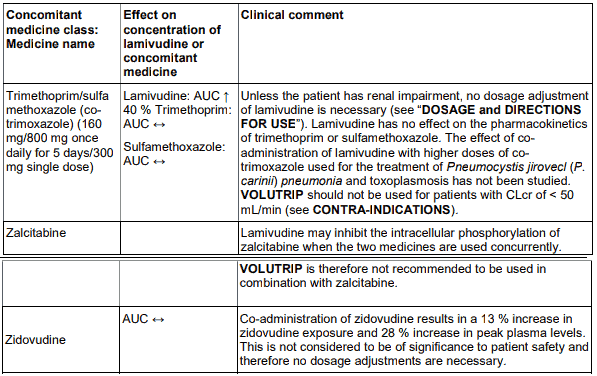
The likelihood of interactions is low due to the limited metabolism as plasma protein binding and almost complete renal clearance. Zidovudine plasma levels are not significantly altered when coadministered with lamivudine. Zidovudine has no effect on the pharmacokinetics of lamivudine. Lamivudine may inhibit the intracellular phosphorylation of zalcitabine when the two medicines are used concurrently. Lamivudine is therefore not recommended to be used in combination with zalcitabine.
Administration of trimethoprim, a constituent of co-trimoxazole causes an increase in lamivudine plasma levels. However, unless the patient has renal impairment, no dosage adjustment of lamivudine is necessary. Lamivudine has no effect on the pharmacokinetics of co-trimoxazole. The possibility of interactions with other medicines administered concurrently should be considered, particularly when the main route is renal.
No medicine interaction studies have been conducted using VOLUTRIP. As VOLUTRIP contains tenofovir disoproxil fumarate and lamivudine, any interactions that have been identified with these individual medicines may occur with VOLUTRIP. Important medicine interaction information for VOLUTRIP is summarised in Tables 1, 2 and 3. The medicine interactions described are based on studies conducted with tenofovir disoproxil fumarate or lamivudine as individual medicines, or are potential medicine interactions. While the tables include potentially significant interactions, they are not all inclusive. Based on the results of in vitro experiments and the known elimination pathway of tenofovir, the potential for CYP450-mediated interactions involving tenofovir with other medicines is low.
An interaction with trimethoprim, a constituent of co-trimoxazole, causes a 40% increase in lamivudine exposure at therapeutic doses. This does not require dose adjustment unless the patient also has renal impairment. Administration of co-trimoxazole with the lamivudine/zidovudine combination in patients with renal impairment should be carefully assessed.
Renally eliminated medicines
Tenofovir, as in VOLUTRIP, is primarily excreted by the kidneys by a combination of glomerular filtration and active tubular secretion.
Co-administration of VOLUTRIP with medicines that are eliminated by active tubular secretion may increase serum concentrations of either tenofovir or the co-administered medicines due to competition for this elimination pathway. Medicines that decrease renal function may also increase serum concentrations of tenofovir, as in VOLUTRIP.
Tenofovir
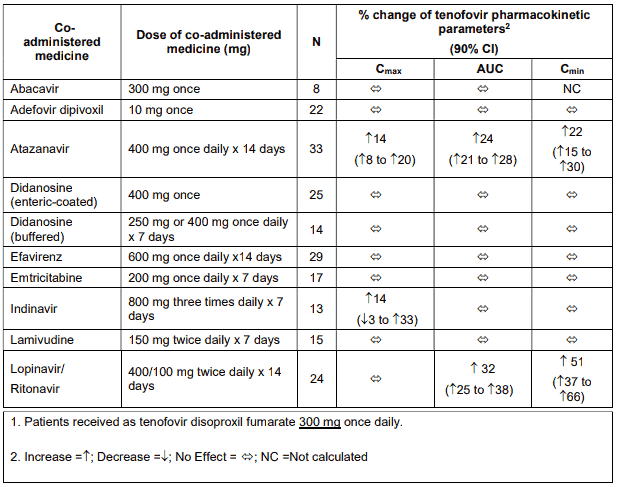
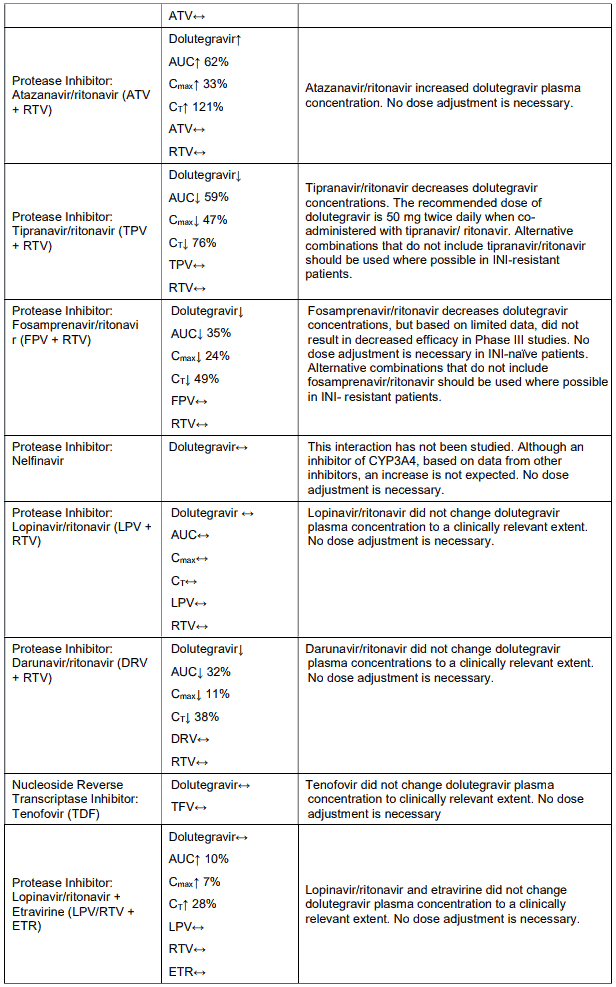
Tenofovir has been evaluated in healthy volunteers in combination with abacavir, adefovir dipivoxil, atazanavir, didanosine, efavirenz, indinavir, lamivudine, lopinavir/ritonavir, methadone, oral contraceptives and ribavirin. Tables 1 and 2 summarise pharmacokinetic effects of co-administered medicine on tenofovir pharmacokinetics and effects of tenofovir on the pharmacokinetics of coadministered medicine.
When administered with multiple doses of tenofovir, the Cmax and AUC of didanosine 400 mg increased significantly. The mechanism of this interaction is unknown. When didanosine 250 mg enteric-coated capsules were administered with tenofovir, systemic exposures to didanosine were similar to those seen with the 400 mg enteric-coated capsules alone under fasted conditions.
Table 1. Medicine interactions: Changes in pharmacokinetic parameters for Tenofovir1 in the presence of co-administered medicines:
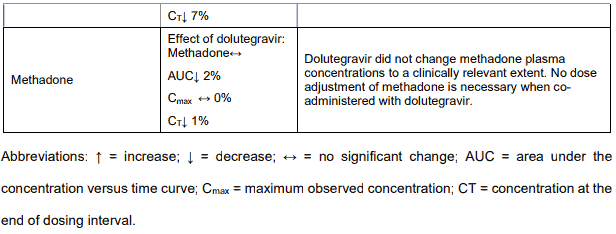
Following multiple dosing to HIV-negative patients receiving either chronic methadone maintenance therapy, oral contraceptives, or single doses of ribavirin, steady-state tenofovir pharmacokinetics were similar to those observed in previous studies, indicating a lack of clinically significant medicine interactions between these medicines and tenofovir disoproxil fumarate.
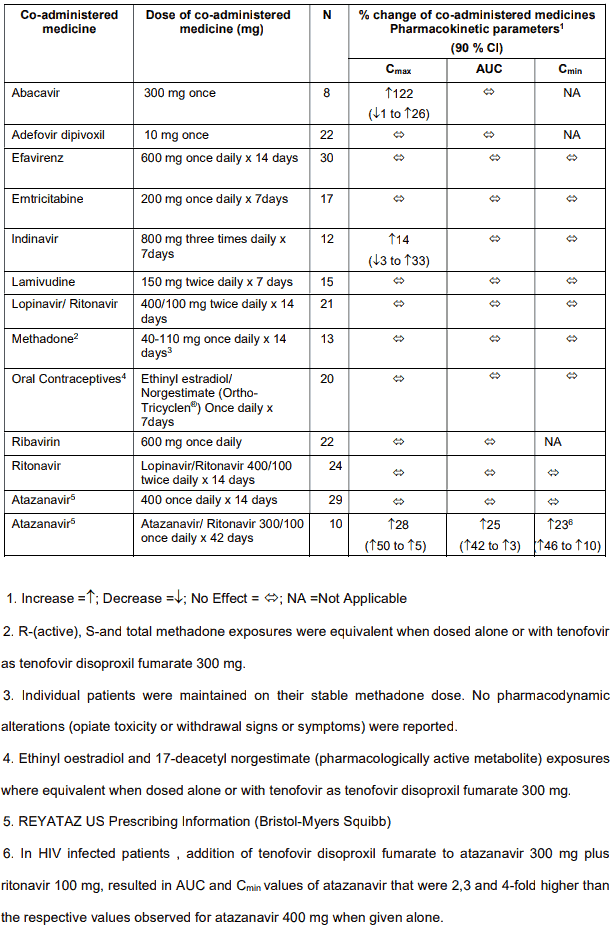
Table 2. Medicine interactions: Changes in pharmacokinetic parameters for co-administered medicine in the presence of tenofovir:
Lamivudine
The likelihood of metabolic interactions is low due to limited metabolism and plasma protein binding and almost complete renal clearance.
Zidovudine plasma levels are not significantly altered when co-administered with VOLUTRIP. Zidovudine has no effect on the pharmacokinetics of VOLUTRIP.
Co-administration of zidovudine results in a 13% increase in zidovudine exposure and 28% increase in peak plasma levels. This is not considered to be of significance to patient safety and therefore no dosage adjustments are necessary.
Table 3. Medicine interactions study reports with lamivudine:
VOLUTRIP may inhibit the intracellular phosphorylation of zalcitabine when the two medicines are used concurrently. VOLUTRIP is therefore nor recommended to be used in combination with zalcitabine.
Administration of trimethoprim, a constituent of co-trimoxazole causes an increase in VOLUTRIP plasma levels. Unless the patient has renal impairment, no dosage adjustment of VOLUTRIP is necessary. VOLUTRIP has no effect on the pharmacokinetics of co-trimoxazole.
The possibility of interactions with other medicines administered concurrently should be considered, particularly when the main route is renal.
The co-administration of VOLUTRIP with etravirine (ETR) is not recommended unless the patient is also receiving concomitant atazanavir + ritonavir (ATV + RTV), lopinavir + ritonavir (LPV + RTV) or darunavir + ritonavir (DRV + RTV).
Dolutegravir
Rifampicin decreases the blood levels of dolutegravir. A supplementary dose of dolutegravir should be given to patients taking VOLUTRIP. There is evidence that the concentration of isoniazid is increased by dolutegravir, as contained in VOLUTRIP.
Effect of dolutegravir as in VOLUTRIP on the pharmacokinetics of other medicines: In vitro, dolutegravir as in VOLUTRIP demonstrated no direct, or weak inhibition (lC50 > 50 µM) of the enzymes cytochrome P450 (CYP)1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A, uridine diphosphate glucuronosyl transferase (UGT)1A1 or UGT2B7, or the transporters Pgp, BCRP, OATP1B1, OATP1B3, OCT1 or MRP2.
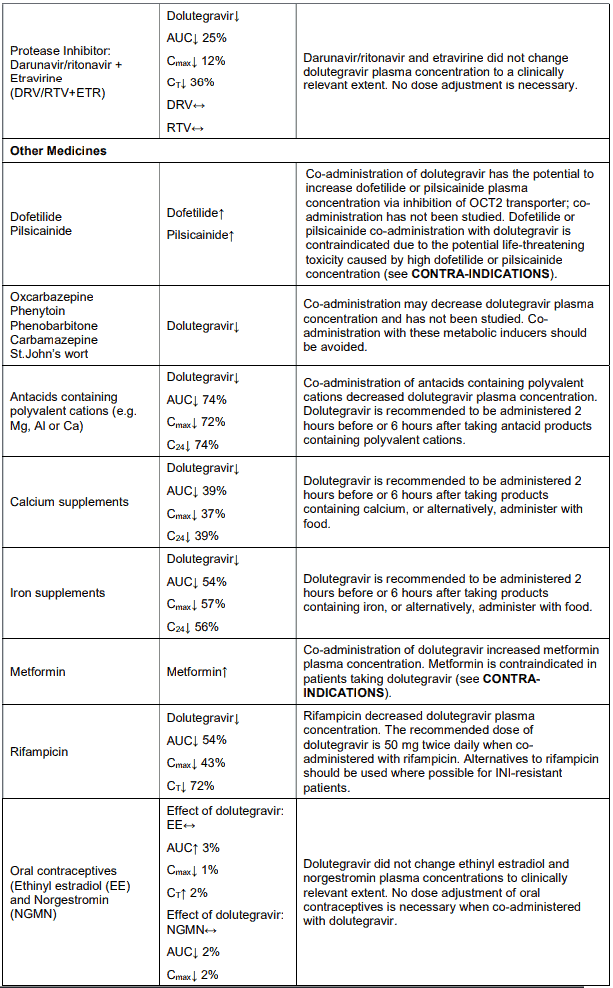
In vitro, dolutegravir as in VOLUTRIP did not induce CYP1A2, CYP2B6 or CYP3A4. In vivo, dolutegravir as in VOLUTRIP did not have an effect on midazolam, a CYP3A4 probe. Based on these data, dolutegravir as in VOLUTRIP is not expected to affect the pharmacokinetics of medicines that are substrates of these enzymes or transporters (e.g., reverse transcriptase and protease inhibitors, opioid analgesics, antidepressants, statins, azole antifungals (such as fluconazole, itraconazole, clotrimazole), proton pump inhibitors (such as esomeprazole, lansoprazole, omeprazole), anti-erectile dysfunction medicines (such as sildenafil, tadalafil, vardenafil), aciclovir, valaciclovir, sitagliptin, adefovir). In medicines interaction study reports, dolutegravir as in VOLUTRIP did not have a clinically relevant effect on the pharmacokinetics of the following: tenofovir, methadone, efavirenz, lopinavir, atazanavir, darunavir, etravirine, fosamprenavir, rilpivirine, telaprevir and oral contraceptives containing norgestimate and ethinyl estradiol.
In vitro, dolutegravir as in VOLUTRIP inhibited the renal organic cation transporter 2 (OCT2). Based on this report, dolutegravir as in VOLUTRIP may increase plasma concentrations of medicines in which excretion is dependent upon OCT2 (dofetilide, metformin) (see Table 4: Medicine Interactions – Other Medicines).
Effect of other medicines on the pharmacokinetics of dolutegravir as in VOLUTRIP: Dolutegravir as in VOLUTRIP is eliminated mainly through metabolism by UGT1A1. Dolutegravir as in VOLUTRIP is also a substrate of UGT1A3, UGT1A9, CYP3A4, Pgp, and BCRP; therefore medicines that induce those enzymes may theoretically decrease dolutegravir plasma concentration and reduce the therapeutic effect of dolutegravir as in VOLUTRIP.
Co-administration of dolutegravir as in VOLUTRIP and other medicines that inhibit UGT1A1, UGT1A3, UGT1A9, CYP3A4, and/or Pgp may increase dolutegravir plasma concentration (see Table 4).
Efavirenz, nevirapine, rifampicin and tipranavir in combination with ritonavir each reduced the plasma concentrations of dolutegravir significantly and require dolutegravir dose adjustment to 50 mg twice daily. Etravirine also reduced plasma concentrations, but the effect of etravirine was mitigated by coadministration of the CYP3A4 inhibitors lopinavir/ritonavir, darunavir/ritonavir and is expected to be mitigated by atazanavir/ritonavir. Therefore no dolutegravir as in VOLUTRIP dose adjustment is necessary when co-administered with etravirine and either Iopinavir/ritonavir, darunavir/ritonavir, or atazanavir/ritonavir. Another inducer, fosamprenavir in combination with ritonavir decreased plasma concentrations of dolutegravir but does not require a dosage adjustment of dolutegravir as in VOLUTRIP. Caution is warranted and clinical monitoring is recommended when these combinations are given in INI-resistant patients (see below table: Medicine Interactions – HIV-1 Antiviral Medicines). A medicine interaction study with the UGT1A1 inhibitor, atazanavir, did not result in a clinically meaningful increase in the plasma concentrations of dolutegravir. Tenofovir, ritonavir, lopinavir/ritonavir, darunavir/ritonavir, rilpivirine, bocepravir, telaprevir, prednisone, rifabutin, and omeprazole had no or a minimal effect on dolutegravir pharmacokinetics, therefore no dolutegravir as in VOLUTRIP dose adjustment is required when co-administered with these medicines.
Table 4. Medicine interactions:
VOLUTRIP should not be co-administered with polyvalent cation-containing antacids. VOLUTRIP is recommended to be administered 2 hours before or 6 hours after these medicines (see INTERACTIONS).
Metformin concentrations may be increased by VOLUTRIP. Metformin is contra-indicated in patients taking VOLUTRIP (see CONTRA-INDICATIONS).
4.6. Pregnancy and lactation
Pregnancy
VOLUTRIP is contraindicated in pregnancy and lactation. Neural tube defects have been noted in an observational study in humans, where DTG-bases regimens were used at the time of conception and early pregnancy, (see CONTRA-INDICATIONS).
Tenofovir, dolutegravir and lamivudine were shown to cross the placenta in reproductive toxicity studies in animals. Late onset neurological disorders, including seizures, have been observed in children who have been exposed to nucleoside analogues in utero such as tenofovir and lamivudine, (see Mitochondrial Dysfunction under WARNINGS AND SPECIAL PRECAUTIONS).
VOLUTRIP should not be prescribed in women who plan to become pregnant. Women of childbearing age should not use VOLUTRIP unless they are using highly effective contraception. Treatment with VOLUTRIP should not be initiated without a medically supervised negative pregnancy test. This test should be repeated at frequent intervals during treatment with VOLUTRIP; and especially in the event that pregnancy is suspected.
Lactation
Mothers breastfeeding their infants should not use VOLUTRIP. Lamivudine is excreted in human milk at similar concentrations to those found in serum; tenofovir is excreted in breast milk and it is not known whether dolutegravir is excreted in human milk.
4.8. Undesirable effects
VOLUTRIP can have side effects.
Lamivudine
The following side effects have been reported during therapy for HIV disease with VOLUTRIP tablets alone and in combination with other antiretrovirals.
Blood and the lymphatic system disorders
Less frequent: Anaemia, neutropenia, thrombocytopaenia
Frequency unknown: pure red cell aplasia.
Metabolism and nutrition disorders
Frequent: Hyperlactataemia
Less frequent: Lactic acidosis, lipodystrophy (redistribution/accumulation of body fat).
Nervous system disorders
Frequent: Headache, insomnia
Less frequent: Peripheral neuropathy (or paraesthesia), late onset neurological disorders in children exposed in utero
Gastrointestinal disorders
Frequent: Nausea, vomiting; upper abdominal pain or cramps; diarrhoea; stomatitis
Less frequent: Pancreatitis, elevations in serum amylase
Hepato-biliary disorders
Less frequent: Transient rises in liver enzymes (AST, ALT)
Skin and subcutaneous tissue disorders
Frequent: Rash, alopecia
Musculoskeletal, connective tissue and bone disorders
Frequent: Arthralgia, muscle disorders
Less frequent: Rhabdomyolysis, decrease in bone mineral density, osteopenia, fractures
General disorders and administrative site conditions
Frequent: Fatigue, malaise, fever
Tenofovir disoproxil fumarate
Immune system disorders
Less frequent: allergic reaction
Gastrointestinal disorders
Frequent: Abdominal pain, anorexia, dyspepsia, flatulence
Less frequent: Increased amylase, pancreatitis
Hepato-biliary disorders
Less frequent: Increased liver enzymes, hepatitis
Metabolism and nutrition disorders
Frequency unknown: Hypophosphatemia, lactic acidosis
Renal and urinary disorders
Frequent: Renal insufficiency, renal failure, proximal tubulopathy, proteinuria, increased creatinine, acute tubular necrosis, nephrogenic diabetes insipidus
Respiratory, thoracic and mediastinal disorders
Frequency unknown: Dyspnoea
Dolutegravir
Immune system disorders
Less frequent: Hypersensitivity, immune reconstitution syndrome
Psychiatric disorders
Frequent: Insomnia
Nervous system disorders
Frequent: Headache, dizziness, abnormal dreams
Gastrointestinal disorders
Frequent: Nausea, diarrhoea
Less frequent: Vomiting, flatulence, upper abdominal pain
Frequency unknown: Abdominal pain, abdominal discomfort
Hepatobiliary disorders
Frequency unknown: Hepatitis
Skin and subcutaneous tissue disorders
Frequent: Rash, pruritus
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.