VOTRIENT Film-coated tablet Ref.[7503] Active ingredients: Pazopanib
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, other antineoplastic agents, protein kinase inhibitors
ATC code: L01XE11
Mechanism of action
Pazopanib is an orally administered, potent multi-target tyrosine kinase inhibitor (TKI) of vascular endothelial growth factor receptors (VEGFR) -1, -2, and -3, platelet-derived growth factor (PDGFR) -α and -β, and stem cell factor receptor (c-KIT), with IC50 values of 10, 30, 47, 71, 84 and 74 nM, respectively. In preclinical experiments, pazopanib dose-dependently inhibited ligand-induced auto-phosphorylation of VEGFR-2, c-Kit and PDGFR-receptors in cells. In vivo, pazopanib inhibited VEGF-induced VEGFR-2 phosphorylation in mouse lungs, angiogenesis in various animal models, and the growth of multiple human tumour xenografts in mice.
Pharmacogenomics
In a pharmacogenetic meta-analysis of data from 31 clinical studies of pazopanib administered either as monotherapy or in combination with other agents, ALT >5 x ULN (NCI CTC Grade 3) occurred in 19% of HLA-B*57:01 allele carriers and in 10% of non-carriers. In this dataset, 133/2235 (6%) of the patients carried the HLA-B*57:01 allele (see section 4.4).
Clinical studies
Renal cell carcinoma (RCC)
The safety and efficacy of pazopanib in RCC were evaluated in a randomised, double-blind, placebo-controlled multicentre study. Patients (N=435) with locally advanced and/or metastatic RCC were randomised to receive pazopanib 800 mg once daily or placebo. The primary objective of the study was to evaluate and compare the two treatment arms for progression-free survival (PFS) and the principle secondary endpoint was overall survival (OS). The other objectives were to evaluate the overall response rate and duration of response.
From the total of 435 patients in this study, 233 patients were treatment-naïve and 202 were second-line patients who had received one prior IL-2 or INF-based therapy. The performance status (ECOG) was similar between the pazopanib and placebo groups (ECOG 0: 42% vs. 41%, ECOG 1: 58% vs. 59%). The majority of patients had either favourable (39%) or intermediate (54%), MSKCC (Memorial Sloan Kettering Cancer Centre)/Motzer prognostic factors. All patients had clear cell histology or predominantly clear cell histology. Approximately half of all patients had 3 or more organs involved in their disease and most patients had the lung (74%), and/or lymph nodes (54%) as a metastatic location for disease at baseline.
A similar proportion of patients in each arm were treatment-naïve and cytokine pre-treated (53% and 47% in pazopanib arm, 54% and 46% in placebo arm). In the cytokine pre-treated subgroup, the majority (75%) had received interferon-based treatment.
Similar proportions of patients in each arm had prior nephrectomy (89% and 88% in the pazopanib and placebo arms, respectively) and/or prior radiotherapy (22% and 15% in the pazopanib and placebo arms, respectively.
The primary analysis of the primary endpoint PFS is based on disease assessment by independent radiological review in the entire study population (treatment-naïve and cytokine pre-treated).
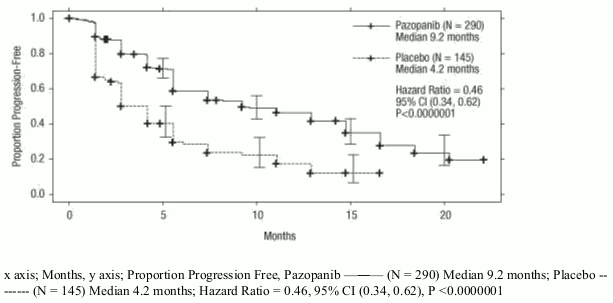
Table 4. Overall efficacy results in RCC by independent assessment (VEG105192):
| Endpoints/Study population | Pazopanib | Placebo | HR (95% CI) | P value (one-sided) |
|---|---|---|---|---|
| PFS | ||||
| Overall* ITT | N=290 | N=145 | ||
| Median (months) | 9.2 | 4.2 | 0.46 (0.34, 0.62) | <0.0000001 |
| Response rate | N=290 | N=145 | ||
| % (95% CI) | 30 (25.1,35.6) | 3 (0.5, 6.4) | - | <0.001 |
HR = hazard ratio; ITT = intent to treat; PFS = progression-free survival
* treatment-naïve and cytokine pre-treated populations
Figure 1. Kaplan-Meier curve for progression-free survival by independent assessment for the overall population (treatment-naïve and cytokine pre-treated populations) (VEG105192):
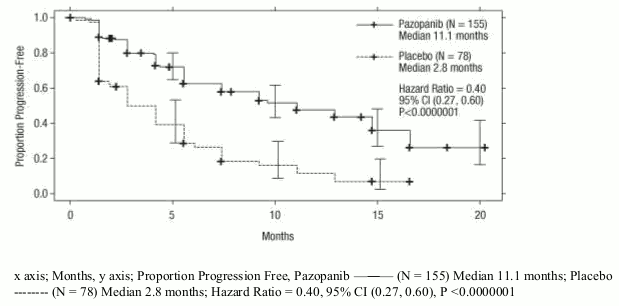
Figure 2. Kaplan-Meier curve for progression-free survival by independent assessment for the treatment-naïve population (VEG105192):
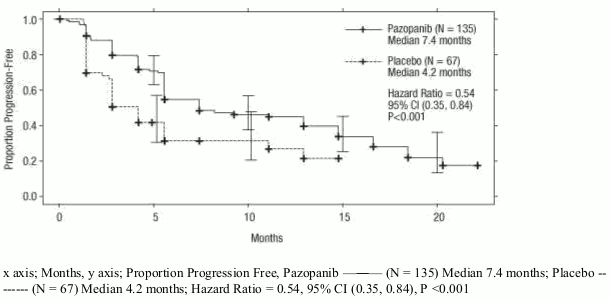
Figure 3. Kaplan-Meier Curve for progression-free survival by independent assessment for the cytokine pre-treated population (VEG105192):
For patients who responded to treatment, the median time to response was 11.9 weeks and the median duration of response was 58.7 weeks as per independent review (VEG105192).
The median overall survival (OS) data at the protocol-specified final survival analysis were 22.9 months and 20.5 months [HR=0.91 (95% CI: 0.71, 1.16; p=0.224)] for patients randomised to the pazopanib and placebo arms, respectively. The OS results are subject to potential bias as 54% of patients in the placebo arm also received pazopanib in the extension part of this study following disease progression. Sixty-six per cent of placebo patients received post-study therapy compared to 30% of pazopanib patients.
No statistical differences were observed between treatment groups for Global Quality of Life using EORTC QLQ-C30 and EuroQoL EQ-5D.
In a Phase II study of 225 patients with locally recurrent or metastatic clear cell renal cell carcinoma, objective response rate was 35% and median duration of response was 68 weeks, as per independent review. Median PFS was 11.9 months.
The safety, efficacy and quality of life of pazopanib versus sunitinib was evaluated in a randomised, open-label, parallel group Phase III non-inferiority study (VEG108844).
In VEG108844, patients (N=1110) with locally advanced and/or metastatic RCC who had not received prior systemic therapy, were randomised to receive either pazopanib 800 mg once daily continuously or sunitinib 50 mg once daily in 6-week cycles of dosing with 4 weeks on treatment followed by 2 weeks without treatment.
The primary objective of this study was to evaluate and compare PFS in patients treated with pazopanib to those treated with sunitinib. Demographic characteristics were similar between the treatment arms. Disease characteristics at initial diagnosis and at screening were balanced between the treatment arms with the majority of patients having clear cell histology and Stage IV disease.
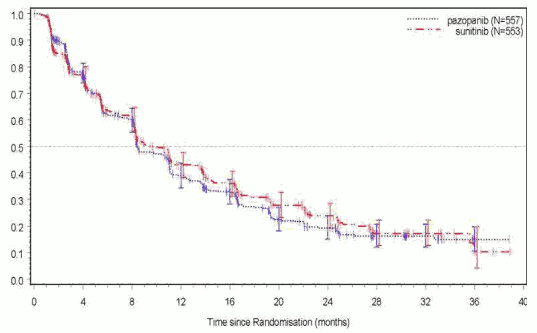
VEG108844 achieved its primary endpoint of PFS and demonstrated that pazopanib was non-inferior to sunitinib, as the upper bound of the 95% CI for the hazard ratio was less than the protocol-specified non-inferiority margin of 1.25. Overall efficacy results are summarised in Table 5.
Table 5. Overall efficacy results (VEG108844):
| Endpoint | Pazopanib N=557 | Sunitinib N=553 | HR (95% CI) |
|---|---|---|---|
| PFS | |||
| Overall | |||
| Median (months) | 8.4 | 9.5 | 1.047 |
| (95% CI) | (8.3, 10.9) | (8.3, 11.0) | (0.898, 1.220) |
| Overall Survival | |||
| Median (months) | 28.3 | 29.1 | 0.915a |
| (95% CI) | (26.0, 35.5) | (25.4, 33.1) | (0.786, 1.065) |
HR = hazard ratio; PFS = progression-free survival; a P value = 0.245 (2-sided)
Figure 4. Kaplan-Meier Curve for progression-free survival by independent assessment for the overall population (VEG108844):
Subgroup analyses of PFS were performed for 20 demographic and prognostic factors. The 95% confidence intervals for all subgroups include a hazard ratio of 1. In the three smallest of these 20 subgroups, the point estimate of the hazard ratio exceeded 1.25; i.e. in subjects with no prior nephrectomy (n=186, HR=1.403, 95% CI (0.955, 2.061)), baseline LDH >1.5 x ULN (n=68, HR=1.72, 95% CI (0.943, 3.139)), and MSKCC: poor risk (n=119, HR=1.472, 95% CI (0.937, 2.313)).
Soft-tissue sarcoma (STS)
The efficacy and safety of pazopanib in STS were evaluated in a pivotal Phase III randomised, double-blind, placebo-controlled multicentre study (VEG110727). A total of 369 patients with advanced STS were randomised to receive pazopanib 800 mg once daily or placebo. Importantly, only patients with selective histological subtypes of STS were allowed to participate to the study, therefore efficacy and safety of pazopanib can only be considered established for those subgroups of STS and treatment with pazopanib should be restricted to such STS subtypes.
The following tumour types were eligible: Fibroblastic (adult fibrosarcoma, myxofibrosarcoma, sclerosing epithelioid fibrosarcoma, malignant solitary fibrous tumours), so-called fibrohistiocytic (pleomorphic malignant fibrous histiocytoma [MFH], giant cell MFH, inflammatory MFH), leiomyosarcoma, malignant glomus tumours, skeletal muscles (pleomorphic and alveolar rhabdomyosarcoma), vascular (epithelioid hemangioendothelioma, angiosarcoma), uncertain differentiation (synovial, epithelioid, alveolar soft part, clear cell, desmoplastic small round cell, extra-renal rhabdoid, malignant mesenchymoma, PEComa, intimal sarcoma), malignant peripheral nerve sheath tumours, undifferentiated soft tissue sarcomas not otherwise specified (NOS) and other types of sarcoma (not listed as ineligible).
The following tumour types were not eligible: Adipocytic sarcoma (all subtypes), all rhabdomyosarcoma that were not alveolar or pleomorphic, chondrosarcoma, osteosarcoma, Ewing tumours/primitive neuroectodermal tumours (PNET), GIST, dermofibromatosis sarcoma protuberans, inflammatory myofibroblastic sarcoma, malignant mesothelioma and mixed mesodermal tumours of the uterus. Of note, patients with adipocytic sarcoma were excluded from the pivotal Phase III study as in a preliminary Phase II study (VEG20002) activity (PFS at week 12) observed with pazopanib in adipocytic did not meet the prerequisite rate to allow further clinical testing.
Other key eligibility criteria of the VEG110727 study were: histological evidence of high or intermediate grade malignant STS and disease progression within 6 months of therapy for metastatic disease, or recurrence within 12 months of (neo) -/adjuvant therapy.
Ninety-eight percent (98%) of subjects received prior doxorubicin, 70% prior ifosfamide, and 65% of subjects had received at least three or more chemotherapeutic agents prior to study enrolment.
Patients were stratified by the factors of WHO performance status (WHO PS) (0 or 1) at baseline and the number of lines of prior systemic therapy for advanced disease (0 or 1 vs. 2+). In each treatment group, there was a slightly greater percentage of subjects in the 2+ lines of prior systemic therapy for advanced disease (58% and 55%, respectively, for placebo and pazopanib treatment arms) compared with 0 or 1 lines of prior systemic therapy (42% and 45%, respectively, for placebo and pazopanib treatment arms). The median duration of follow-up of subjects (defined as date of randomisation to date of last contact or death) was similar for both treatment arms (9.36 months for placebo [range 0.69 to 23.0 months] and 10.04 months for pazopanib [range 0.2 to 24.3 months].
The primary objective of the study was progression-free survival (PFS assessed by independent radiological review); the secondary endpoints included overall survival (OS), overall response rate and duration of response.
Table 6. Overall efficacy results in STS by independent assessment (VEG110727):
| Endpoints/study population | Pazopanib | Placebo | HR (95% CI) | P value (two-sided) |
|---|---|---|---|---|
| PFS | ||||
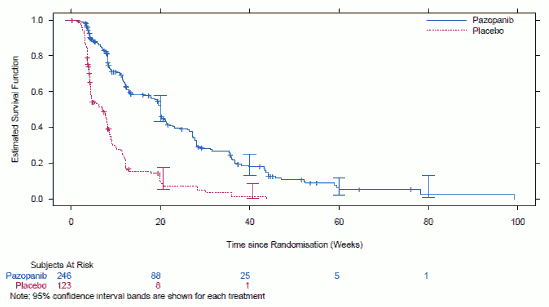
| Overall ITT | N=246 | N=123 | ||
| Median (weeks) | 20.0 | 7.0 | 0.35 (0.26, 0.48) | <0.001 |
| Leiomyosarcoma | N=109 | N=49 | ||
| Median (weeks) | 20.1 | 8.1 | 0.37 (0.23, 0.60) | <0.001 |
| Synovial sarcoma subgroups | N=25 | N=13 | ||
| Median (weeks) | 17.9 | 4.1 | 0.43 (0.19, 0.98) | 0.005 |
| 'Other STS' subgroups | N=112 | N=61 | ||
| Median (weeks) | 20.1 | 4.3 | 0.39 (0.25, 0.60) | <0.001 |
| OS | ||||
| Overall ITT | N=246 | N=123 | ||
| Median (months) | 12.6 | 10.7 | 0.87 (0.67, 1.12) | 0.256 |
| Leiomyosarcoma* | N=109 | N=49 | ||
| Median (months) | 16.7 | 14.1 | 0.84 (0.56, 1.26) | 0.363 |
| Synovial sarcoma subgroups* | N=25 | N=13 | ||
| Median (months) | 8.7 | 21.6 | 1.62 (0.79, 3.33) | 0.115 |
| "Other STS" subgroups* | N=112 | N=61 | ||
| Median (months) | 10.3 | 9.5 | 0.84 (0.59, 1.21) | 0.325 |
| Response rate (CR+PR) | ||||
| % (95% CI) | 4 (2.3, 7.9) | 0 (0.0, 3.0) | ||
| Duration of response | ||||
| Median (weeks) (95% CI) | 38.9 (16.7, 40.0) | |||
HR = hazard ratio; ITT = intent to treat; PFS = progression-free survival; CR = complete response; PR = partial response. OS = overall survival
* Overall survival for the respective STS histological subgroups (leiomyosarcoma, synovial sarcoma and "Other" STS) should be interpreted with caution due to the small number of subjects and wide confidence intervals
A similar improvement in PFS based on investigator assessments was observed in the pazopanib arm compared with the placebo arm (in the overall ITT population HR: 0.39; 95% CI, 0.30 to 0.52, p<0.001).
Figure 5. Kaplan-Meier Curve for Progression-Free Survival in STS by Independent Assessment for the Overall Population (VEG110727):
No significant difference in OS was observed between the two treatment arms at the final OS analysis performed after 76% (280/369) of the events had occurred (HR 0.87, 95% CI 0.67, 1.12 p=0.256).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Votrient in all subsets of the paediatric population in treatment of kidney and renal pelvis carcinoma (excluding nephroblastoma, nephroblastomatosis, clear cell sarcoma, mesoblastic nephroma, renal medullary carcinoma and rhabdoid tumour of the kidney) (see section 4.2 for information on paediatric use).
The European Medicines Agency has deferred the obligation to submit the results of studies with Votrient in one or more subsets of the paediatric population in the treatment of rhabdomyosarcoma, non-rhabdomyosarcoma soft tissue sarcoma and Ewing sarcoma family of tumours (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
Upon oral administration of a single pazopanib 800 mg dose to patients with solid tumours, maximum plasma concentration (Cmax) of approximately 19 ± 13 μg/ml was obtained after median 3.5 hours (range 1.0-11.9 hours) and an AUC0-∞ of approximately 650 ± 500 μg.h/ml was obtained. Daily dosing results in 1.23- to 4-fold increase in AUC0-T.
There was no consistent increase in AUC or Cmax at pazopanib doses above 800 mg.
Systemic exposure to pazopanib is increased when administered with food. Administration of pazopanib with a high-fat or low-fat meal results in an approximately 2-fold increase in AUC and Cmax. Therefore, pazopanib should be administered at least two hours after food or at least one hour before food (see section 4.2).
Administration of a pazopanib 400 mg crushed tablet increased AUC(0-72) by 46% and Cmax by approximately 2 fold and decreased tmax by approximately 2 hours compared to administration of the whole tablet. These results indicate that the bioavailability and the rate of pazopanib oral absorption are increased after administration of the crushed tablet relative to administration of the whole tablet (see section 4.2).
Distribution
Binding of pazopanib to human plasma protein in vivo was greater than 99% with no concentration dependence over the range of 10-100 μg/ml. In vitro studies suggest that pazopanib is a substrate for P-gp and BCRP.
Biotransformation
Results from in vitro studies demonstrated that metabolism of pazopanib is mediated primarily by CYP3A4, with minor contributions from CYP1A2 and CYP2C8. The four principle pazopanib metabolites account for only 6% of the exposure in plasma. One of these metabolites inhibits the proliferation of VEGF-stimulated human umbilical vein endothelial cells with a similar potency to that of pazopanib, the others are 10- to 20-fold less active. Therefore, activity of pazopanib is mainly dependent on parent pazopanib exposure.
Elimination
Pazopanib is eliminated slowly with a mean half-life of 30.9 hours after administration of the recommended dose of 800 mg. Elimination is primarily via faeces with renal elimination accounting for <4% of the administered dose.
Special populations
Renal impairment
Results indicate that less than 4% of an orally administered pazopanib dose is excreted in the urine as pazopanib and metabolites. Results from population pharmacokinetic modelling (data from subjects with baseline CLCR values ranging from 30.8 ml/min to 150 ml/min) indicated that renal impairment is unlikely to have clinically relevant effect on pazopanib pharmacokinetics. No dose adjustment is required in patients with creatinine clearance above 30 ml/min. Caution is advised in patients with creatinine clearance below 30 ml/min as there is no experience of pazopanib in this patient population (see section 4.2).
Hepatic impairment
Mild:
The median steady-state pazopanib Cmax and AUC(0-24) in patients with mild abnormalities in hepatic parameters (defined as either normal bilirubin and any degree of ALT elevation or as an elevation of bilirubin up to 1.5 x ULN regardless of the ALT value) after administration of 800 mg once daily are similar to the median in patients with normal hepatic function (see Table 7). 800 mg pazopanib once daily is the recommended dose in patients with mild abnormalities of serum liver tests (see section 4.2).
Moderate:
The maximally tolerated pazopanib dose (MTD) in patients with moderate hepatic impairment (defined as an elevation of bilirubin >1.5 x to 3 x ULN regardless of the ALT values) was 200 mg once daily. The median steady-state Cmax and AUC(0-24) values after administration of 200 mg pazopanib once daily in patients with moderate hepatic impairment were approximately 44% and 39%, of the corresponding median values after administration of 800 mg once daily in patients with normal hepatic function, respectively (see Table 7).
Based on safety and tolerability data, the dose of pazopanib should be reduced to 200 mg once daily in subjects with moderate hepatic impairment (see section 4.2).
Severe:
The median steady-state Cmax and AUC(0-24) values after administration of 200 mg pazopanib once daily in patients with severe hepatic impairment were approximately 18% and 15%, of the corresponding median values after administration of 800 mg once daily in patients with normal hepatic function. Based on the diminished exposure and limited hepatic reserve pazopanib is not recommended in patients with severe hepatic impairment (defined as total bilirubin >3 X ULN regardless of any level of ALT) (see section 4.2).
Table 7. Median steady-state pazopanib pharmacokinetics measured in subjects with hepatic impairment:
| Group | Investigated dose | Cmax (μg/ml) | AUC(0-24) (μg x hr/ml) | Recommended dose |
|---|---|---|---|---|
| Normal hepatic function | 800 mg OD | 52.0 (17.1-85.7) | 888.2 (345.5-1482) | 800 mg OD |
| Mild HI | 800 mg OD | 33.5 (11.3-104.2) | 774.2 (214.7-2034.4) | 800 mg OD |
| Moderate HI | 200 mg OD | 22.2 (4.2-32.9) | 256.8 (65.7-487.7) | 200 mg OD |
| Severe HI | 200 mg OD | 9.4 (2.4-24.3) | 130.6 (46.9-473.2) | Not recommended |
OD - once daily
Preclinical safety data
The preclinical safety profile of pazopanib was assessed in mice, rats, rabbits and monkeys. In repeat dose studies in rodents, effects in a variety of tissues (bone, teeth, nail beds, reproductive organs, haematological tissues, kidney and pancreas) appear related to the pharmacology of VEGFR inhibition and/or disruption of VEGF signalling pathways, with most effects occurring at plasma exposure levels below those observed in the clinic. Other observed effects include body weight loss, diarrhoea and/or morbidity that were either secondary to local gastrointestinal effects caused by high local mucosal medicinal product exposure (monkeys) or pharmacological effects (rodents). Proliferative hepatic lesions (eosinophilic foci and adenoma) were seen in female mice at exposures 2.5 times human exposure based on AUC.
In juvenile toxicity studies, when pre-weaning rats were dosed from day 9 post partum through to day 14 post partum, pazopanib caused mortalities and abnormal organ growth/maturation in kidney, lung, liver and heart, at a dose approximately 0.1 times the clinical exposure based on AUC in adult humans. When post-weaning rats were dosed from day 21 post partum to day 62 post partum, toxicological findings were similar to adult rats at comparable exposures. Human paediatric patients are at increased risk for bone and teeth effects as compared to adults, as these changes, including inhibition of growth (shortened limbs), fragile bones and remodelling of teeth, were present in juvenile rats at ≥10 mg/kg/day (equal to approximately 0.1-0.2 times the clinical exposure based on AUC in adult humans) (see section 4.4).
Reproductive, fertility and teratogenic effects
Pazopanib has been shown to be embryotoxic and teratogenic when administered to rats and rabbits at exposures more than 300-fold lower than the human exposure (based on AUC). Effects included reduced female fertility, increased pre- and post-implantation loss, early resorptions, embryo lethality, decreased foetal body weight and cardiovascular malformation. Decreased corpora lutea, increased cysts and ovarian atrophy have also been noted in rodents. In a rat male fertility study, there was no effect on mating or fertility, but decreased testicular and epididymal weights were noted with reductions in sperm production rates, sperm motility, and epididymal and testicular sperm concentrations observed at exposures 0.3 times human exposure based on AUC.
Genotoxicity
Pazopanib did not cause genetic damage when tested in genotoxicity assays (Ames assay, human peripheral lymphocyte chromosome aberration assay and rat in vivo micronucleus). A synthetic intermediate in manufacture of pazopanib, which is also present in the final drug substance in low amounts, was not mutagenic in the Ames assay but genotoxic in the mouse lymphoma assay and in vivo mouse micronucleus assay.
Carcinogenicity
In two-year carcinogenicity studies with pazopanib, there were increased numbers of liver adenomas noted in mice and duodenal adenocarcinomas noted in rats. Based on the rodent-specific pathogenesis and mechanism for these findings, they are not considered to represent an increased carcinogenic risk for patients taking pazopanib.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.