VYJUVEK Suspension and gel for gel Ref.[115167] Active ingredients: Beremagene geperpavec
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Krystal Biotech Netherlands, B.V., Atrium Gebouw, Strawinskylaan 3051, Amsterdam 1077 ZX, Netherlands
4.1. Therapeutic indications
Vyjuvek is indicated for the treatment of wounds in patients with dystrophic epidermolysis bullosa (DEB) with mutation(s) in the collagen type VII alpha 1 chain (COL7A1) gene, from birth.
4.2. Posology and method of administration
Vyjuvek should be initiated by healthcare professionals experienced in the management of patients with dystrophic epidermolysis bullosa.
Posology
Vyjuvek is applied cutaneously to wound(s) once a week in small droplets in a grid-like pattern, approximately 1-cm by 1-cm apart. All wounds may not be possible to be treated at each treatment visit.
The recommended total maximum weekly dosing for children from birth up to 3 years old is 1 mL (2×109 PFU). The recommended total maximum weekly dosing for children above 3 years of age, adolescents, and adults is 2 mL (4×109 PFU).
Vyjuvek should be applied to wounds until they are closed before selecting new wound(s) to treat. Weekly treatment of previously treated wounds should be prioritised if they re-open. If no wounds are present, Vyjuvek should not be administered.
The table below provides a reference on dose per approximate size of the wound in children, adolescents, and adults.
Table 1. Dose by wound area:
| Wound area (cm2)* | Dose (PFU)a | Volume (mL) |
|---|---|---|
| <20 | <4×108 | <0.2 |
| 20 to <40 | 4×108 to <8×108 | 0.2 to <0.4 |
| 40 to 60 | 8×108 to <1.2×109 | 0.4 to <0.6 |
| 60 to <200 | 1.2×109 to <4×109 | 0.6 to <2 |
PFU = plaque forming units.
a The maximum dose in children below 3 years of age is 1 mL (2×109 PFU)
If a dose is missed, Vyjuvek should be administered as soon as possible, and weekly dosing should be resumed thereafter.
Special populations
Elderly population
No dose adjustment is required in patients ≥ 65 years old.
Method of administration
Precaution to be taken before manipulating or administering the product
This medicine contains genetically modified organisms (see section 4.4). During preparation, administration, and disposal, appropriate precautions must be taken. Personal protective equipment (e.g., gloves, mask, and eye protection) should be worn when handling Vyjuvek.
Pregnant women should not prepare or administer Vyjuvek and should avoid direct contact with the treated wounds, or dressings from the treated wounds (see section 6.6).
Administration
For cutaneous use on wounds only.
Prior to cutaneous use the suspension and gel must be thawed, and the suspension must be mixed into the gel in a pharmacy setting. For detailed instructions on preparation, shelf life after mixing, administration, measures to take in case of accidental exposure, logistics, and disposal of Vyjuvek, see sections 6.3 and 6.6.
A health care professional (HCP) should apply Vyjuvek, either at a healthcare professional setting (e.g., clinic) or the home setting. If deemed appropriate by the healthcare professional, trained patients or caregivers may also apply Vyjuvek.
Wounds should be gently cleaned prior to cutaneous administration using a product that does not contain a virucidal agent. Medicinal products and ointments at the wound area should be removed and the wound should be cleansed prior to Vyjuvek administration to ensure no reduction in its activity (see section 4.5).
Table 2. Steps for administration:
| Step 1. The Vyjuvek syringe should be primed prior to the initial application by pulling the plunger down and pushing it upwards, so that a small droplet of Vyjuvek forms at the tip of the syringe. | |
| Step 2. Vyjuvek should be applied to the selected wound, in small droplets approximately 1-cm by 1-cm apart (width of a fingertip) with only the droplet touching the wound. Only the gel should contact the skin. The tip of the syringe should not touch the skin to prevent the contamination of the gel in the syringe. | 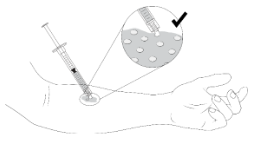 |
| Step 3. Once Vyjuvek has been administered to the wound, a hydrophobic dressing should be applied. The dressing should be cut to a size slightly larger than the wound but may vary upon patient preference. Once the Vyjuvek droplets are covered by the hydrophobic dressing, a thin even layer of Vyjuvek will form within the wound. | 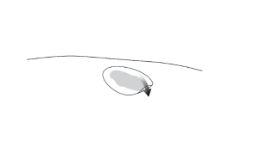 |
| Step 4. The standard dressing should be cut to a size larger than the hydrophobic dressing. The standard dressing will be placed over the hydrophobic dressing to prevent dissemination of the gel to other areas of the body or close contacts. |  |
The dressing should be left in place for approximately 24 hours after Vyjuvek application. Once the Vyjuvek dressings are removed, the patient may continue with their standard of care.
Vyjuvek should continue to be administered weekly until the wounds are closed. If previously treated wounds re-open, Vyjuvek should be applied again. If no wounds are present, Vyjuvek should not be administered.
4.9. Overdose
No case of overdose of Vyjuvek has been reported. Symptomatic and supportive treatment, as deemed necessary by the treating healthcare professional, is advised in case of overdose.
6.3. Shelf life
Unopened cartons:
2 years when stored in the freezer.
After thawing:
If a freezer is not available, the carton(s) may be stored in a refrigerator (2°C to 8°C) for up to 1 month.
Once stored in the refrigerator, the medicinal product should not be re-frozen.
After mixing:
Chemical and physical in-use stability has been demonstrated for 168 hours (7 days) at 2-8°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 24 hours at 2 to 8°C, unless mixing has taken place in controlled and validated aseptic conditions.
Syringes can be stored at room temperature for up to 8 hours.
Transport conditions for mixed product:
Transport mixed product at 2-8°C to site of administration.
6.4. Special precautions for storage
Unopened cartons:
Store frozen at -15°C to -25°C. Transport frozen (< -20°C).
Keep the vials in the carton prior to thawing in order to protect from light.
After thawing and mixing:
For storage conditions after thawing and after mixing of the medicinal product, see section 6.3.
6.5. Nature and contents of container
Each carton of Vyjuvek contains one vial of suspension and one vial of gel.
Suspension:
1 mL extractable volume containing 5×109 PFU in a cyclo-olefin copolymer vial with a thermoplastic elastomer closure and green cap.
Gel:
1.5 mL fill volume in a separate Type-1 glass vial with a bromobutyl elastomer stopper and blue cap.
6.6. Special precautions for disposal and other handling
Precautions to be taken before handling or administering the medicinal product
This medicine contains genetically modified organisms (see section 4.4). During preparation, administration, and disposal, appropriate precautions must be taken. Personal protective equipment (e.g., gloves, mask, and eye protection) should be worn when handling Vyjuvek.
HCPs or carers who are pregnant should not administer Vyjuvek and should not come into direct contact with treated wounds, or all material that has been in contact with treated wounds.
Preparation prior to administration
Follow the steps below for Vyjuvek preparation.
Each carton contains one vial of suspension (1 mL extractable volume containing 5×109 PFU) and one vial of excipient gel (1.5 mL).
Concentration of the medicinal product is 2×109 PFU/mL after mixing.
Table 5. Preparation steps prior to administration:
| Step 1 | Step 2 | |
| Before use, frozen vials must be removed from the carton and left at room temperature. (Step 1). Once the vials are thawed (for approximately 30 minutes), they cannot be re-frozen. (Step 2) Visually inspect the suspension vial. The suspension may contain white to off-white particulates that are inherent to the product. The suspension may vary in colour from opalescent yellow to colourless. Do not use this medication if you notice any discolouration. Visually inspect the gel vial. The gel is a clear, colourless, viscous gel. Do not use the gel if you notice any particulates or discolouration. Gently invert the suspension vial 4-5 times to mix the contents. Remove the caps from the vials and clean each vial stopper with an alcohol pad. Allow them to dry. | 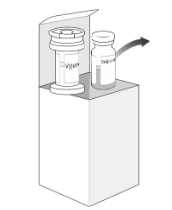 | 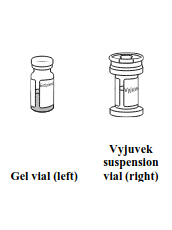 |
| Using an aseptic technique, withdraw 1 mL of thawed suspension (Step 1) using a 3 mL syringe and needle (e.g., 16G or 18G). Transfer 1 mL of thawed suspension into the thawed gel vial. (Step 2). | 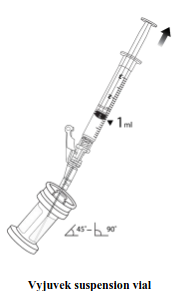 | 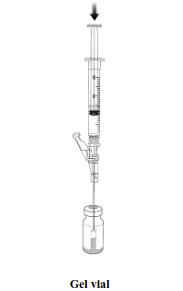 |
| Without removing the needle from the gel vial, pull the needle so it is above the liquid, remove 1 mL of air (air pocket) to vent the gel vial following the addition of the 1 mL of Vyjuvek suspension, and only then remove the syringe and needle and discard them. The vial with the combined suspension and gel will be referred to as the Vyjuvek vial for the remainder of these instructions. | 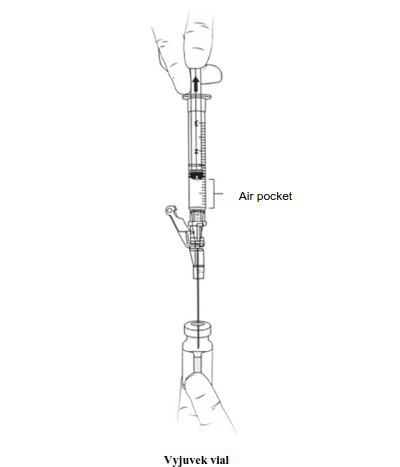 | |
| Place an alcohol pad on the gel vial stopper and shake the vial vigorously by hand for at least 10 seconds. The excipient gel should incorporate the suspension to form a homogeneous gel. Visually inspect the Vyjuvek vial. The gel containing the active substance may contain white to off- white particulates that are inherent to the product. The mixed product, like the suspension, may vary in colour from opalescent yellow to colourless. Do not use this medication if you notice any discolouration. | 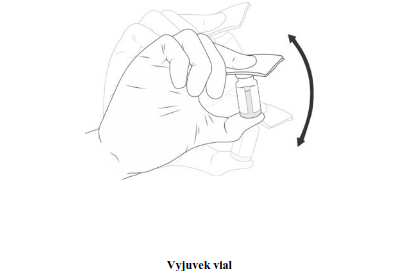 | |
| Step 1 | Step 2 | |
| Connect a new needle (e.g., 16G or 18G) to a 1 mL syringe and slowly withdraw 0.5 mL of Vyjuvek (Step 1). Do not invert the vial to withdraw the Vyjuvek syringe. Without removing the needle from the vial, lift the tip of the needle above the Vyjuvek and disconnect the syringe, leaving the needle within the vial stopper (Step 2). An air pocket may form, this is normal. | 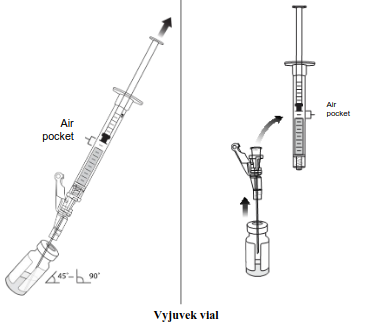 | |
| Gently manipulate the plunger up and down to remove the air pocket. DO NOT flick the syringe to remove the air pocket. Small bubbles may remain, this is normal. | 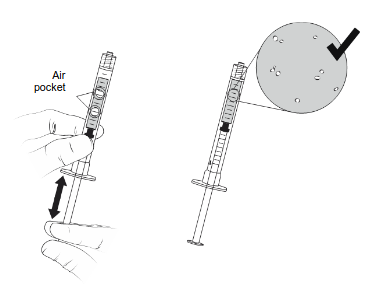 | |
| Cap the syringe and set aside. |  | |
| Obtain the next 1 mL syringe and connect it to the needle in the gel vial stopper and withdraw 0.5 mL of Vyjuvek, remove the air pocket and cap the syringe. Extractable volume is 2.0 mL (4×109 PFU). Repeat as applicable based on the recommended posology. Label the syringe with patient’s ID, name of the product, lot number, EXP date, and storage conditions. Avoid covering the syringe marks needed for administration. Place the capped Vyjuvek syringes into a sealable plastic bag. Label the plastic bag with patient’s ID, name of the product, lot number, EXP date, and storage conditions. No more than 2 mL (four 0.5 mL syringes) can be used in the same week as this is the maximum weekly dose. |  | |
Place a sealable plastic bag with Vyjuvek syringes into an appropriate insulated tertiary container (“outer container”) to maintain a transport temperature of 2°C to 8°C suitable for transport and in order to protect from light.
The outer container needs to be fully closed for transportation.
Open the outer container designed for transportation of prepared Vyjuvek syringes only at the site of administration.).
Reception and storage at administration site
After receipt of the outer container, store the outer container in a secure, room-temperature location that is clean, out of reach of children, and free from potential contamination.
Only the person responsible for the administration should open the outer container.
The person responsible for the administration should check that the outer container is intact and there are no signs of leakage before use (see section 4.2).
Measures to take in case of accidental exposure
In case of accidental exposure local guidance for pharmaceutical waste must be followed.
All surfaces that may have come in contact with beremagene geperpavec must be cleaned and all spills must be disinfected with a virucidal agent such as 70% isopropyl alcohol, 6% hydrogen peroxide or <0.4% ammonium chloride.
In the event of an accidental exposure through a splash to the eyes or mucous membranes, flush with clean water for at least 5 minutes.
In the event of exposure to intact skin or needlestick injury, clean the affected area thoroughly with soap and water and/or a disinfectant.
Precautions to be taken for the disposal of the medicinal product
Any unused medicinal product or waste material (e.g., vial, syringe, needle, cleaning materials) that that may have come in contact with Vyjuvek should be disposed of in compliance with local guidance for pharmaceutical waste.
Disinfect dressings with a virucidal agent, such as 70% isopropyl alcohol, 6% hydrogen peroxide or <0.4% ammonium chloride, and dispose of the disinfected dressings in a separate sealed plastic bag in household waste or according to local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.