VYLEESI Solution for injection Ref.[9967] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Bremelanotide is a melanocortin receptor (MCR) agonist that nonselectively activates several receptor subtypes with the following order of potency: MC1R, MC4R, MC3R, MC5R, MC2R. At therapeutic dose levels, binding to MC1R and MC4R is most relevant. Neurons expressing MC4R are present in many areas of the central nervous system (CNS). The mechanism by which VYLEESI improves HSDD in women is unknown. The MC1R is expressed on melanocytes; binding at this receptor leads to melanin expression and increased pigmentation.
12.2. Pharmacodynamics
Transient Increases in Blood Pressure
In an open-label ambulatory blood pressure monitoring study of 127 premenopausal women receiving VYLEESI once daily, there was a mean increase of 1.9 mmHg (95% CI: 1.0 to 2.7) in daytime systolic blood pressure (SBP) and a mean increase of 1.7 mmHg (95% CI: 0.9 to 2.4) in daytime diastolic blood pressure (DBP) after 8 days of dosing. The increase in SBP and DBP was transient with a mean peak effect in SBP of 2.8 mmHg between 4 to 8 hours post-dose and 2.7 mmHg for DBP at 0 to 4 hours post-dose. The increase in BP after 8 days of dosing was accompanied by a simultaneous and transient mean decrease in heart rate of 0.5 beats per minute (95% CI: -1.6 to -0.7). The SBP and DBP values 12 to 24-hours post-dose were similar to the pre-dose values [see Warnings and Precautions (5.1)].
Alcohol Interaction
A placebo-controlled, randomized, double-blind, three-period, three-way crossover study was conducted to assess the safety of a single intranasal 20 mg dose of bremelanotide co-administered with alcohol in 12 healthy male and 12 healthy female subjects. Intranasal bremelanotide or placebo spray was administered 10 minutes after consumption of placebo drink or 0.6 g/kg ethanol (equivalent of three 12 ounce cans of beer containing 5% alcohol content, three 5 ounce glasses of wine containing 12% alcohol content, or three 1.5 ounce shots of 80-proof spirit in a 70 kg person).
The 20 mg intranasal dose achieves a 2.5-fold higher mean Cmax than that of VYLEESI. Alcohol consumption had no effect on the pharmacokinetic profile of bremelanotide. The incidence of flushing was higher with bremelanotide plus ethanol compared to ethanol alone, but similar to the incidence with bremelanotide alone. The incidence of headache was higher with bremelanotide plus ethanol compared to bremelanotide alone, but similar to the incidence with ethanol alone. The incidence of other adverse reactions was similar across the treatment groups. The incidence of abnormal orthostatic blood pressure reductions was comparable between the bremelanotide plus ethanol group and the ethanol alone group. No participants discontinued due to adverse reactions.
Cardiac Electrophysiology
A 20 mg intranasal dose of bremelanotide does not prolong the QTc interval to any clinically relevant extent.
12.3. Pharmacokinetics
Following subcutaneous administration of VYLEESI, the mean plasma Cmax and AUC of bremelanotide are 72.8 ng/mL and 276 hr*ng/mL, respectively. Mean plasma concentrations of bremelanotide increase in a less than dose proportional manner in the dose range of 0.3 to 10 mg, with mean Cmax levels reaching a plateau at the 7.5 mg subcutaneous dose level (approximately 4.3 times the maximum recommended dose).
Absorption
Bremelanotide median Tmax is approximately 1.0 hour (range: 0.5-1.0 hours) in plasma. The absolute bioavailability of bremelanotide following subcutaneous administration of VYLEESI was about 100%. The site of subcutaneous administration (abdomen and thigh) had no significant effect on the systemic exposure to bremelanotide.
Distribution
Twenty-one percent of bremelanotide binds to human serum protein. The mean (SD) volume of distribution after a single subcutaneous administration of VYLEESI is 25.0 ± 5.8 L.
Elimination
Following a single subcutaneous administration of VYLEESI, mean terminal half-life of bremelanotide is approximately 2.7 hours (range: 1.9–4.0 hours) and the mean (± SD) clearance (CL/F) is 6.5 ± 1.0 L/hr.
Metabolism
As a peptide with 7 amino acids, the primary metabolic pathway of bremelanotide involves multiple hydrolyses of the amide bond of the cyclic peptide.
Excretion
Following administration of a radiolabeled dose, 64.8% of the total radioactivity was recovered in urine and 22.8% in feces.
Specific Populations
Patients with Renal Impairment
Following a single subcutaneous dose of VYLEESI, bremelanotide exposure (AUC) increased 1.2-fold in patients with mild (eGFR, 60 to 89 mL/min/1.73 m²) renal impairment, 1.5-fold in patients with moderate (eGFR, 30 to 59 mL/min/1.73 m²) renal impairment, and 2-fold in patients with severe (eGFR, <30 mL/min/1.73 m²) renal impairment [see Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Following a single subcutaneous dose of VYLEESI, bremelanotide exposure (AUC0-inf) increased 1.2-fold in patients with mild (Child-Pugh A; score of 5-6) hepatic impairment and 1.7-fold in patients with moderate (Child-Pugh B; score of 7-9) hepatic impairment [see Use in Specific Populations (8.7)]. The effect of severe hepatic impairment on the pharmacokinetics of bremelanotide was not studied.
Drug Interaction Studies
Potential for VYLEESI to Influence the Pharmacokinetics of Other Drugs
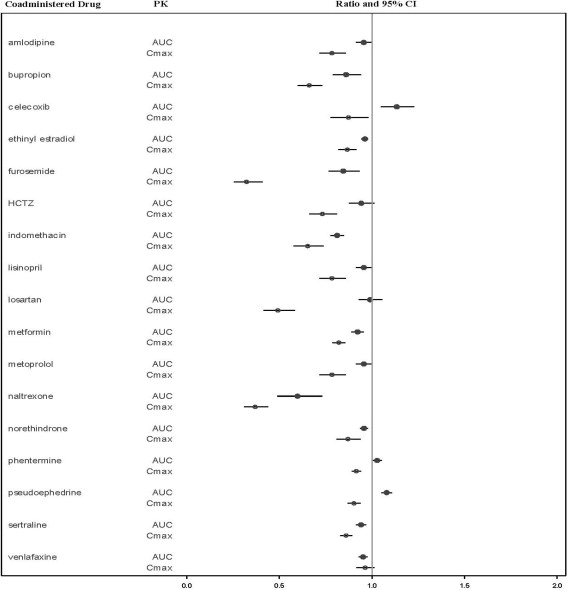
VYLEESI may reduce the rate and extent of absorption of concomitantly administered oral medications, likely due to slowing gastric motility. In clinical pharmacology studies, VYLEESI did not affect the absorption of the tested orally administered concomitant medications to any clinically relevant degree, except for naltrexone and indomethacin [see Drug Interactions (7)].
The effects of bremelanotide on the pharmacokinetics of other drugs are summarized below as change relative to the other drug administered alone (test/reference) (Figure 1).
Figure 1. Effects of Bremelanotide 1.75 mg SC on the Pharmacokinetic Exposures of Orally Administered Medications:
Note: AUC = area under the plasma concentration-time curve; BMT = bremelanotide; CI = confidence interval; Cmax = maximum plasma concentration; SC = subcutaneous.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
There were no significant increases in tumor incidence in two-year carcinogenicity studies with intranasal administration (0.5, 2.5, and 5 mg/animal/day) of bremelanotide to male and female rats, and subcutaneous administration (3, 9, and 15 mg/kg/day) to male and female mice. Multiples of exposure were calculated based on average Cmax at the high dose over the course of the study and were 1.1-fold and 111-fold the human Cmax for rats and mice, respectively.
Mutagenesis
Bremelanotide was not genotoxic or mutagenic in a battery of tests, including the in vitro bacterial reverse mutation assay, the in vitro chromosomal aberration test in Chinese Hamster Ovary cells, and the in vivo mouse micronucleus assay.
Impairment of Fertility
There were no effects on fertility in male (75 mg/kg/day, approximately 375 times the human AUC) or female (150 mg/kg/day, approximately 760 times the human AUC) mice following subcutaneous administration.
14. Clinical Studies
The efficacy of VYLEESI for the treatment of HSDD in premenopausal women was evaluated in two identical, Phase 3, randomized, double-blind, placebo-controlled trials: NCT02333071 and NCT02338960 (Study 1 and Study 2). Both trials included premenopausal women with acquired, generalized HSDD of at least 6 months' duration. All patients in heterosexual relationships were required to use an effective form of contraception. A majority of patients (74% in Study 1 and 67% in Study 2) reported HSDD with concomitant decreased arousal. The trials consisted of two phases: a Core Study Phase (24-week placebo-controlled, double-blind treatment period) and an uncontrolled, 52-week Open-label Extension Study Phase.
Study participants were randomized to subcutaneous injections of VYLEESI 1.75 mg (n=635) or placebo (n=632), self-administered by an autoinjector on an as-needed basis. Patients were instructed to administer the drug approximately 45 minutes prior to anticipated sexual activity. Patients were not to administer more than one dose within a 24-hour period and no more than twelve doses per month. Trial participants were mostly Caucasian (86%) or Black (12%). The mean age of study participants was 39 years old (range 19 to 56 years old); the mean duration in a monogamous relationship was 12 years, and the mean duration of HSDD was approximately 4 years. Across the two trials, the median number of VYLEESI injections was 10 in the 24-week double-blind treatment period and 12 during the uncontrolled open-label extension. Most patients used VYLEESI two to three times per month and no more than once a week.
Study 1 and Study 2 had the following co-primary efficacy endpoints:
- Change from baseline to end of study (EOS) in the Desire domain from the Female Sexual Function Index (FSFI) (Questions 1 and 2). Question 1 asks patients "Over the past 4 weeks, how often did you feel sexual desire or interest?", with responses ranging from 1 (almost never or never) to 5 (almost always or always). Question 2 asks patients "Over the past 4 weeks, how would you rate your level (degree) of sexual desire or interest?", with responses ranging from 1 (very low or none at all) to 5 (very high). The FSFI Desire domain score was calculated by adding the patient’s responses to these two questions then multiplying that sum by 0.6. The FSFI Desire Domain score ranged from 1.2 to 6. An increase in the FSFI Desire domain score over time denotes improvement in sexual desire.
- Change from baseline to EOS in the score for feeling bothered by low sexual desire as measured by the Female Sexual Distress Scale – Desire/Arousal/Orgasm Question 13 (FSDS-DAO Q13). This question asks patients, "How often did you feel: Bothered by low sexual desire?" Patients assessed their sexual distress over a 30-day recall period and responded on a scale of 0 (never) to 4 (always). A decrease in the FSDS-DAO Q13 score over time denotes improvement in the level of distress associated with low sexual desire.
EOS is defined as the patient’s last study visit during the double-blind treatment period. For patients who completed the double-blind treatment period, the EOS visit occurred at Week 24.
Efficacy results for these co-primary endpoints from Study 1 and Study 2 are summarized in Table 2 and Table 3. In both studies, VYLEESI showed a statistically significant increase in the FSFI Desire Domain score and a statistically significant decrease in the FSDS-DAO Q13 score from baseline to the EOS visit compared to placebo. The magnitude of the treatment differences was similar in both studies.
Table 2. Efficacy Results for the FSFI-Desire Domain Score in Premenopausal HSDD Patients in Study 1 and Study 2 (MITT* Population):
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| VYLEESI 1.75 mg (N=313) | Placebo (N=315) | VYLEESI 1.75 mg (N=282) | Placebo (N=288) | |
| Mean Baseline (SD)1 | 2.1 (0.9) | 2.0 (0.8) | 2.0 (0.8) | 2.1 (0.8) |
| Mean Change from Baseline (SD) | 0.5 (1.1) | 0.2 (1.0) | 0.6 (1.0) | 0.2 (0.9) |
| Median Change from Baseline | 0.6 | 0 | 0.6 | 0 |
| p-value2 | 0.0002 | <0.0001 | ||
1 FSFI Desire score range: 1.2 to 6.0, with higher scores indicating greater desire.
2 p-value from unadjusted Wilcoxon rank-sum test.
* MITT: modified intent to treat defined as all patients who were randomized, used at least one dose of double-blind study drug, and had at least one double-blind follow-up visit. However, one VYLEESI patient and one placebo patient in Study 1 and two placebo patients in Study 2 did not have either a baseline or EOS efficacy measurement and change from baseline could not be calculated. Therefore, N = the number of patients in the MITT population with an evaluable change measurement.
Table 3. Efficacy Results for the FSDS-DAO Q13 Score in Premenopausal HSDD Patients in Study 1 and Study 2 (MITT* Population):
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| VYLEESI 1.75 mg (N=313) | Placebo (N=314) | VYLEESI 1.75 mg (N=282) | Placebo (N=285) | |
| Mean Baseline (SD)1 | 2.9 (1.0) | 2.8 (0.9) | 2.9 (0.9) | 2.9 (0.9) |
| Mean Change from Baseline (SD) | -0.7 (1.2) | -0.4 (1.1) | -0.7 (1.1) | -0.4 (1.1) |
| Median Change from Baseline | -1 | 0 | -1 | 0 |
| p-value2 | <0.0001 | 0.0053 | ||
1 FSDS-DAO Q13 score range: 0 to 4, with higher scores indicating greater bother.
2 p-value from unadjusted Wilcoxon rank-sum test.
* MITT: modified intent to treat defined as all patients who were randomized, used at least one dose of the double-blind drug and had at least one double-blind follow-up visit. However, one VYLEESI patient and two placebo patients in Study 1 and five placebo patients in Study 2 did not have either a baseline or EOS efficacy measurement and change from baseline could not be calculated. Therefore, N = the number of patients in the MITT population with an evaluable change measurement
Supplementary analyses were conducted to help interpret clinical meaningfulness of the observed score change from baseline to EOS in the FSFI-Desire Domain and FSDS-DAO Q13. These analyses defined responders for each coprimary efficacy endpoint by anchoring change from baseline to EOS with multiple anchor measures. Each anchor analysis considered responders to be those who reported experiencing meaningful change at their EOS visit according to the respective anchor measure.
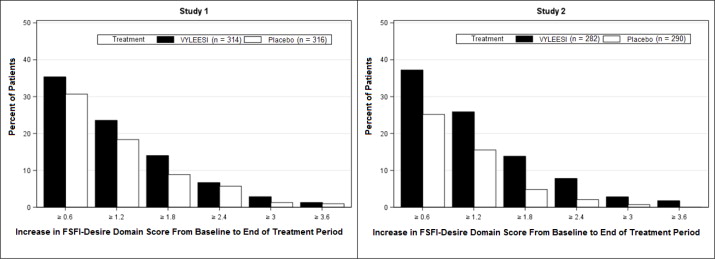
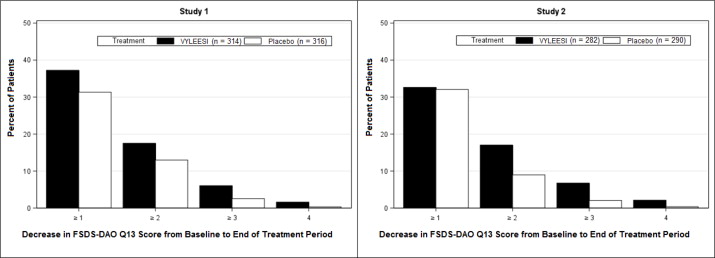
Because a greater percentage of MITT patients in the VYLEESI group prematurely discontinued the 24-week double-blind treatment period compared to placebo patients (40% vs. 13% for Study 1 and 39% vs. 25% for Study 2), an exploratory analysis was performed examining the percentages of patients who were able to complete the treatment period and improved from baseline. Figure 2 displays the percentages of the MITT patients in the two Phase 3 trials who completed the 24-week double-blind treatment period and achieved various levels of increase in the FSFI-Desire Domain Score from baseline (higher scores indicate increased sexual desire). Figure 3 displays the percentages of the MITT patients in the two clinical trials who completed the 24-week double-blind treatment period and achieved various levels of reduction in the FSDS-DAO Q13 score from baseline (higher scores indicate greater reduction in distress).
Figure 2. Percent of Patients (MITT Population) who Completed the 24-Week Double-Blind Treatment Period and Achieved Various Levels of Increases in the FSFI-Desire Domain Score:
Patients who did not complete the double-blind treatment period or were missing baseline scores are not considered to have experienced an increase in FSFI-Desire Domain score at the end of the double-blind treatment period.
Responder threshold: at least 1.2-point increase from baseline in FSFI-Desire Domain score. The threshold was defined for these studies by anchoring change from baseline to end of treatment with multiple anchor measures.
Figure 3. Percent of Patients (MITT Population) who Completed the 24-Week Double-Blind Treatment Period and Achieved Various Levels of Reductions in the FSDS-DAO Q13 Score:
Patients who did not complete the double-blind treatment period or were missing change from baseline scores are not considered to have experienced a decrease in FSDS-DAO Q13 score at the end of the double-blind treatment period.
Responder threshold: at least 1-point decrease from baseline in the FSDS-DAO Q13 score. The threshold was defined for these studies by anchoring change from baseline to end of treatment with multiple anchor measures.
There was no significant difference between treatment groups in the change from baseline to end of study visit in the number of satisfying sexual events (SSEs), a secondary endpoint.
Efficacy results for the number of SSEs are summarized in Table 4.
Table 4. Efficacy Results for the Number of Satisfying Sexual Events in Premenopausal HSDD Patients in Study 1 and Study 2 (MITT* Population):
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| VYLEESI 1.75 mg (N=314) | Placebo (N=316) | VYLEESI 1.75 mg (N=282) | Placebo (N=290) | |
| Mean Baseline (SD) | 0.7 (1.0) | 0.8 (1.1) | 0.8 (1.1) | 0.7 (1.0) |
| Mean Change from Baseline (SD) | 0.0 (1.4) | -0.1 (1.4) | 0.0 (1.3) | 0.0 (1.2) |
| Median Change from Baseline | 0 | 0 | 0 | 0 |
| p-value1 | 0.76 | 0.70 | ||
1 p-value from unadjusted Wilcoxon rank-sum test.
* MITT: modified intent to treat defined as all patients who were randomized, used at least one dose of double-blind drug and had at least 1 double-blind follow-up visit. N = the number of patients in the MITT population.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.