VYONDYS 53 Solution for injection Ref.[10301] Active ingredients:
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
VYONDYS 53 (golodirsen) injection is a sterile, aqueous, preservative-free, concentrated solution for dilution prior to intravenous administration. VYONDYS 53 is a clear to slightly opalescent, colorless liquid, and may contain trace amounts of small, white to off-white amorphous particles. VYONDYS 53 is supplied in single-dose vials containing 100 mg golodirsen (50 mg/mL). VYONDYS 53 is formulated as an isotonic phosphate buffered saline solution with an osmolality of 260 to 320 mOSM and a pH of 7.5. Each milliliter of VYONDYS 53 contains: 50 mg golodirsen; 0.2 mg potassium chloride; 0.2 mg potassium phosphate monobasic; 8 mg sodium chloride; and 1.14 mg sodium phosphate dibasic, anhydrous, in water for injection. The product may contain hydrochloric acid or sodium hydroxide to adjust pH.
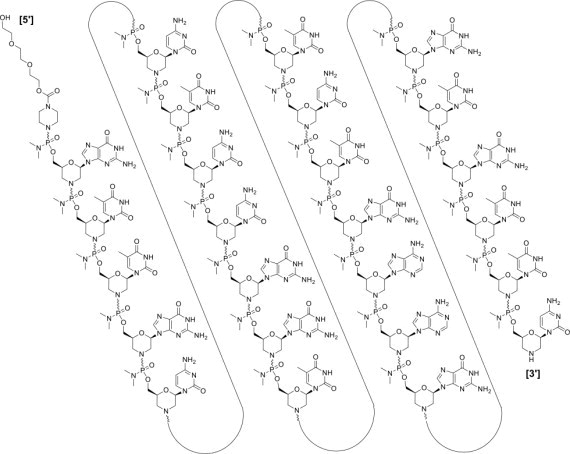
Golodirsen is an antisense oligonucleotide of the phosphorodiamidate morpholino oligomer (PMO) subclass. PMOs are synthetic molecules in which the five-membered ribofuranosyl rings found in natural DNA and RNA are replaced by a six-membered morpholino ring. Each morpholino ring is linked through an uncharged phosphorodiamidate moiety rather than the negatively charged phosphate linkage that is present in natural DNA and RNA. Each phosphorodiamidate morpholino subunit contains one of the heterocyclic bases found in DNA (adenine, cytosine, guanine, or thymine). Golodirsen contains 25 linked subunits. The sequence of bases from the 5' end to 3' end is GTTGCCTCCGGTTCTGAAGGTGTTC. The molecular formula of golodirsen is C305H481N138O112P25 and the molecular weight is 8647.28 daltons.
The structure of golodirsen is:
| Dosage Forms and Strengths |
|---|
|
VYONDYS 53 is a clear to slightly opalescent, colorless liquid, and may contain trace amounts of small, white to off-white amorphous particles, and available as:
|
| How Supplied |
|---|
|
VYONDYS 53 injection is supplied in single dose vials. The solution is a clear to slightly opalescent, colorless liquid, and may contain trace amounts of small, white to off-white amorphous particles. Single-dose vials containing 100 mg/2mL (50 mg/mL) - NDC 60923-465-02 |
Drugs
| Drug | Countries | |
|---|---|---|
| VYONDYS 53 | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.