WEGOVY Solution for injection Ref.[49657] Active ingredients: Semaglutide
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Novo Nordisk A/S, Novo Allé, DK-2880 Bagsværd, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, glucagon-like peptide-1 (GLP-1) analogues
ATC code: A10BJ06
Mechanism of action
Semaglutide is a GLP-1 analogue with 94% sequence homology to human GLP-1. Semaglutide acts as a GLP-1 receptor agonist that selectively binds to and activates the GLP-1 receptor, the target for native GLP-1.
GLP-1 is a physiological regulator of appetite and calorie intake, and the GLP-1 receptor is present in several areas of the brain involved in appetite regulation.
Animal studies show that semaglutide works in the brain through the GLP-1 receptor. Semaglutide has direct effects on areas in the brain involved in homeostatic regulation of food intake in the hypothalamus and the brainstem. Semaglutide may affect the hedonic reward system through direct and indirect effects in brain areas including the septum, thalamus and amygdala.
Clinical studies show that semaglutide reduces energy intake, increases feelings of satiety, fullness and control of eating, reduces feelings of hunger, and frequency and intensity of cravings. In addition, semaglutide reduces the preference for high fat foods.
Semaglutide orchestrates the homeostatic and hedonic contributions with executive function to regulate caloric intake, appetite, reward and food choice.
In addition, in clinical studies semaglutide have shown to reduce blood glucose in a glucose dependent manner by stimulating insulin secretion and lowering glucagon secretion when blood glucose is high. The mechanism of blood glucose lowering also involves a minor delay in gastric emptying in the early postprandial phase. During hypoglycaemia, semaglutide diminishes insulin secretion and does not impair glucagon secretion.
GLP-1 receptors are also expressed in the heart, vasculature, immune system and kidneys. Semaglutide has a beneficial effect on plasma lipids, lowered systolic blood pressure and reduced inflammation in clinical studies. Furthermore, animal studies have shown that semaglutide attenuated the development of atherosclerosis and had an anti-inflammatory action in the cardiovascular system.
Pharmacodynamic effects
Appetite, energy intake and food choice
Semaglutide reduces appetite by increasing feelings of fullness and satiety, while lowering hunger and prospective food consumption. In a phase 1 trial, energy intake during an ad libitum meal was 35% lower with semaglutide compared to placebo after 20 weeks of dosing. This was supported by improved control of eating, less food cravings and a relative lower preference for high fat food. Food cravings were further assessed in STEP 5 by a Control of Eating Questionnaire (CoEQ). At week 104, the estimated treatment difference both for control of cravings and craving of savoury food significantly favoured semaglutide, whereas no clear effect was seen for craving of sweet food.
Fasting and postprandial lipids
Semaglutide 1 mg compared to placebo lowered fasting triglyceride and very low density lipoproteins (VLDL) concentrations by 12% and 21%, respectively. The postprandial triglyceride and VLDL response to a high fat meal was reduced with >40%.
Clinical efficacy and safety
The efficacy and safety of semaglutide for weight management in combination The efficacy and safety of semaglutide for weight management in combination with a reduced calorie intake and increased physical activity were evaluated in four 68 weeks double-blinded randomised placebo-controlled phase 3a trials (STEP 1-4). A total of 4,684 adult patients (2,652 randomised to treatment with semaglutide) were included in these trials. Furthermore, the two-year efficacy and safety of semaglutide compared to placebo were evaluated in a double-blinded randomised placebo-controlled phase 3b trial (STEP 5) including 304 patients (152 in treatment with semaglutide).
Treatment with semaglutide demonstrated superior, clinically meaningful, and sustained weight loss compared with placebo in patients with obesity (BMI ≥30 kg/m²), or overweight (BMI ≥27 kg/m² to <30 kg/m²) and at least one weight-related comorbidity. Furthermore, across the trials, a higher proportion of patients achieved ≥5%, ≥10%, ≥15% and ≥20% weight loss with semaglutide compared with placebo. The reduction in body weight occurred irrespective of the presence of gastrointestinal symptoms such as nausea, vomiting or diarrhoea.
Treatment with semaglutide also showed statistically significant improvements in waist circumference, systolic blood pressure and physical functioning compared to placebo.
Efficacy was demonstrated regardless of age, sex, race, ethnicity, baseline body weight, BMI, presence of type 2 diabetes and level of renal function. Variations in efficacy existed within all subgroups. Relatively greater weight loss was observed in women and in patients without type 2 diabetes as well as in patients with a lower versus higher baseline body weight.
STEP 1: Weight management
In a 68-week double-blind trial, 1,961 patients with obesity (BMI ≥30 kg/m²), or with overweight (BMI ≥27 kg/m² to <30 kg/m²) and at least one weight-related comorbidity were randomised to semaglutide or placebo. All patients were on a reduced-calorie diet and increased physical activity throughout the trial.
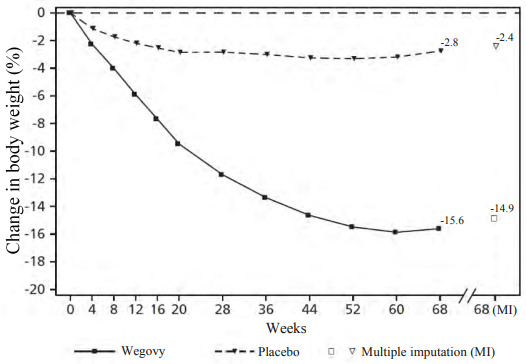
Weight loss occurred early and continued throughout the trial. At end of treatment (week 68), the weight loss was superior and clinically meaningful compared with placebo (see Table 4 and Figure 1). Furthermore, a higher proportion of patients achieved ≥5%, ≥10%, ≥15% and ≥20% weight loss with semaglutide compared with placebo (see Table 4). Among patients with prediabetes at baseline, a higher proportion of patients had a normo-glycaemic status at end of treatment with semaglutide compared to placebo (84.1% vs. 47.8%).
Table 4. STEP 1: Results at week 68:
| Wegovy | Placebo | |
|---|---|---|
| Full analysis set (N) | 1,306 | 655 |
| Body weight | ||
| Baseline (kg) | 105.4 | 105.2 |
| Change (%) from baseline1,2 | -14.9 | -2.4 |
| Difference (%) from placebo1 [95% CI] | -12.4 [-13.4; -11.5]* | - |
| Change (kg) from baseline | -15.3 | -2.6 |
| Difference (kg) from placebo1 [95% CI] | -12.7 [-13.7; -11.7] | - |
| Patients (%) achieving weight loss ≥5%3 | 83.5* | 31.1 |
| Patients (%) achieving weight loss ≥10%3 | 66.1* | 12.0 |
| Patients (%) achieving weight loss ≥15%3 | 47.9* | 4.8 |
| Waist circumference (cm) | ||
| Baseline | 114.6 | 114.8 |
| Change from baseline1 | -13.5 | -4.1 |
| Difference from placebo1 [95% CI] | -9.4 [-10.3; -8.5]* | - |
| Systolic blood pressure (mmHg) | ||
| Baseline | 126 | 127 |
| Change from baseline1 | -6.2 | -1.1 |
| Difference from placebo1 [95% CI] | -5.1 [-6.3; -3.9]* | - |
* p<0.0001 (unadjusted 2-sided) for superiority.
1 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
2 During the trial, randomised treatment was permanently discontinued by 17.1% and 22.4% of patients randomised to semaglutide 2.4 mg and placebo, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for body weight based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -16.9% and -2.4% for semaglutide 2.4 mg and placebo respectively.
3 Estimated from binary regression model based on same imputation procedure as in primary analysis.
Figure 1. STEP 1: Mean change in body weight (%) from baseline to week 68:
Observed values for patients completing each scheduled visit, and estimates with multiple imputations (MI) from retrieved dropouts.
STEP 2: Weight management in patients with type 2 diabetes
In a 68-week, double-blind trial, 1,210 patients with overweight or obesity (BMI ≥27 kg/m²) and type 2 diabetes were randomised to either semaglutide 2.4 mg, semaglutide 1 mg once-weekly or placebo. Patients included in the trial had insufficiently controlled diabetes (HbA1c 7–10%) and were treated with either: diet and exercise alone or 1–3 oral antidiabetic drugs. All patients were on a reduced-calorie diet and increased physical activity throughout the trial.
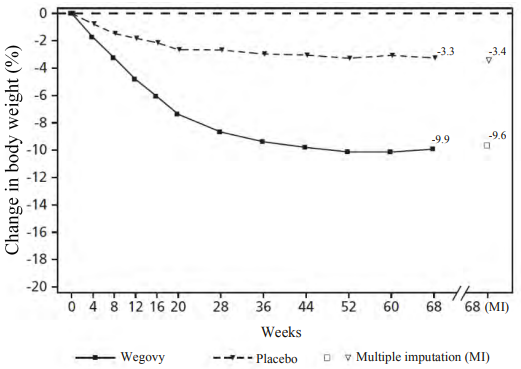
Treatment with semaglutide for 68 weeks resulted in superior and clinically meaningful reduction in body weight and in HbA1c compared to placebo (see Table 5 and Figure 2).
Table 5. STEP 2: Results at week 68:
| Wegovy | Placebo | |
|---|---|---|
| Full analysis set (N) | 404 | 403 |
| Body weight | ||
| Baseline (kg) | 99.9 | 100.5 |
| Change (%) from baseline1,2 | -9.6 | -3.4 |
| Difference (%) from placebo1 [95% CI] | -6.2 [-7.3;-5.2]* | - |
| Change (kg) from baseline | -9.7 | -3.5 |
| Difference (kg) from placebo1 [95% CI] | -6.1 [-7.2;-5.0] | - |
| Patients (%) achieving weight loss ≥5%3 | 67.4* | 30.2 |
| Patients (%) achieving weight loss ≥10%3 | 44.5* | 10.2 |
| Patients (%) achieving weight loss ≥15%3 | 25.0* | 4.3 |
| Waist circumference (cm) | ||
| Baseline | 114.5 | 115.5 |
| Change from baseline1 | -9.4 | -4.5 |
| Difference from placebo1 [95% CI] | -4.9 [-6.0; -3.8]* | - |
| Systolic blood pressure (mmHg) | ||
| Baseline | 130 | 130 |
| Change from baseline1 | -3.9 | -0.5 |
| Difference from placebo1 [95% CI] | -3.4 [-5.6; -1.3]** | - |
| HbA1c (mmol/mol (%)) | ||
| Baseline | 65.3 (8.1) | 65.3 (8.1) |
| Change from baseline1 | -17.5 (-1.6) | -4.1 (-0.4) |
| Difference from placebo1 [95% CI] | -13.5 [-15.5; -11.4] (-1.2 [-1.4; -1.1])* | - - |
* p<0.0001 (unadjusted 2-sided) for superiority; **p<0.05 (unadjusted 2-sided) for superiority
1 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
2 During the trial, randomised treatment was permanently discontinued by 11.6% and 13.9% of patients randomised to semaglutide 2.4 mg and placebo, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for body weight based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -10.6% and -3.1% for semaglutide 2.4 mg and placebo respectively
3 Estimated from binary regression model based on same imputation procedure as in primary analysis.
Figure 2. STEP 2: Mean change in body weight (%) from baseline to week 68:
Observed values for patients completing each scheduled visit, and estimates with multiple imputations (MI) from retrieved dropouts.
STEP 3: Weight management with intensive behavioural therapy
In a 68-week double-blind trial, 611 patients with obesity (BMI ≥30 kg/m²), or with overweight (BMI ≥27 kg/m² to <30 kg/m²) and at least one weight-related comorbidity were randomised to semaglutide or placebo. During the trial, all patients received intensive behavioural therapy (IBT) consisting of a very restrictive diet, increased physical activity and behavioural counselling.
Treatment with semaglutide and IBT for 68 weeks resulted in superior and clinically meaningful reduction in body weight compared to placebo (see Table 6).
Table 6. STEP 3: Results at week 68:
| Wegovy | Placebo | |
|---|---|---|
| Full analysis set (N) | 407 | 204 |
| Body weight | ||
| Baseline (kg) | 106.9 | 103.7 |
| Change (%) from baseline1,2 | -16.0 | -5.7 |
| Difference (%) from placebo1 [95% CI] | -10.3 [-12.0;-8.6]* | - |
| Change (kg) from baseline | -16.8 | -6.2 |
| Difference (kg) from placebo1 [95% CI] | -10.6 [-12.5;-8.8] | - |
| Patients (%) achieving weight loss ≥5%3 | 84.8* | 47.8 |
| Patients (%) achieving weight loss ≥10%3 | 73.0* | 27.1 |
| Patients (%) achieving weight loss ≥15%3 | 53.5* | 13.2 |
| Waist circumference (cm) | ||
| Baseline | 113.6 | 111.8 |
| Change from baseline1 | -14.6 | -6.3 |
| Difference from placebo1 [95% CI] | -8.3 [-10.1; -6.6]* | - |
| Systolic blood pressure (mmHg) | ||
| Baseline | 124 | 124 |
| Change from baseline1 | -5.6 | -1.6 |
| Difference from placebo1 [95% CI] | -3.9 [-6.4; -1.5]* | |
* p<0.005 (unadjusted 2-sided) for superiority
1 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
2 During the trial, randomised treatment was permanently discontinued by 16.7% and 18.6% of patients randomised to semaglutide 2.4 mg and placebo, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for body weight based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -17.6% and -5.0% for semaglutide 2.4 mg and placebo respectively
3 Estimated from binary regression model based on same imputation procedure as in primary analysis.
STEP 4: Sustained weight management
In a 68-week double-blind trial, 902 patients with obesity (BMI ≥30 kg/m²), or with overweight (BMI ≥27 kg/m² to <30 kg/m²) and at least one weight-related comorbidity were included in the trial. All patients were on a reduced-calorie diet and increased physical activity throughout the trial. From week 0 to week 20 (run-in), all patients received semaglutide. At week 20 (baseline), patients who had reached the maintenance dose of 2.4 mg were randomised to continue treatment or switch to placebo. At week 0 (start of run-in period) patients had a mean body weight of 107.2 kg and a mean BMI of 38.4 kg/m².
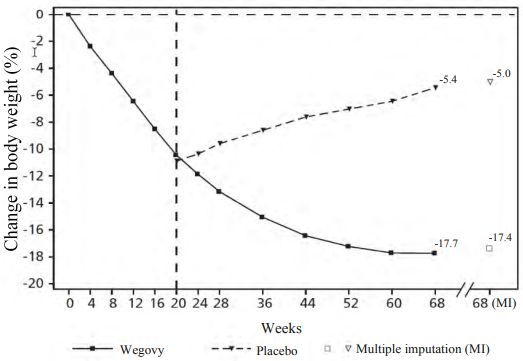
Patients who had reached the maintenance dose of 2.4 mg at week 20 (baseline) and continued treatment with semaglutide for 48 weeks (week 20–68) continued losing weight and had a superior and clinically meaningful reduction in body weight compared to those switched to placebo (see Table 7 and Figure 3). The body weight increased steadily from week 20 to week 68 in patients switching to placebo at week 20 (baseline). Nevertheless, the observed mean body weight was lower at week 68 than at start of the run-in period (week 0) (see Figure 3). Patients treated with semaglutide from week 0 (run-in) to week 68 (end of treatment) achieved a mean change in body weight of 17.4%, with weight loss ≥5% achieved by 87.8%, ≥10% achieved by 78.0%, ≥15% achieved by 62.2% and ≥20% achieved by 38.6% of these patients.
Table 7. STEP 4: Results from week 20 to week 68:
| Wegovy | Placebo | |
|---|---|---|
| Full analysis set (N) | 535 | 268 |
| Body weight | ||
| Baseline1 (kg) | 96.5 | 95.4 |
| Change (%) from baseline1,2,3 | -7.9 | 6.9 |
| Difference (%) from placebo2 [95% CI] | -14.8 [-16.0; -13.5]* | - |
| Change (kg) from baseline | -7.1 | 6.1 |
| Difference (kg) from placebo2 [95% CI] | -13.2 [-14.3; -12.0] | - |
| Waist circumference (cm) | ||
| Baseline | 105.5 | 104.7 |
| Change from baseline1 | -6.4 | 3.3 |
| Difference from placebo2 [95% CI] | -9.7 [-10.9; -8.5]* | - |
| Systolic blood pressure (mmHg) | ||
| Baseline1 | 121 | 121 |
| Change from baseline1,2 | 0.5 | 4.4 |
| Difference from placebo2 [95% CI] | -3.9 [-5.8; -2.0]* | |
* p<0.0001 (unadjusted 2-sided) for superiority,
1 Baseline = week 20
2 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
3 During the trial, randomised treatment was permanently discontinued by 5.8% and 11.6% of patients randomized to semaglutide 2.4 mg and placebo, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for body weight based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -8.1% and 6.5% for semaglutide 2.4 mg and placebo respectively.
Figure 3. STEP 4: Mean change in body weight (%) from week 0 to week 68:
Observed values for patients completing each scheduled visit, and estimates with multiple imputations (MI) from retrieved dropouts
STEP 5: 2-year data
In a 104-week double-blind trial, 304 patients with obesity (BMI ≥30 kg/m²), or with overweight (BMI ≥27 to <30 kg/m²) and at least one weight-related comorbidity, were randomised to semaglutide or placebo. All patients were on a reduced-calorie diet and increased physical activity throughout the trial. At baseline, patients had a mean BMI of 38.5 kg/m², a mean body weight of 106.0 kg.
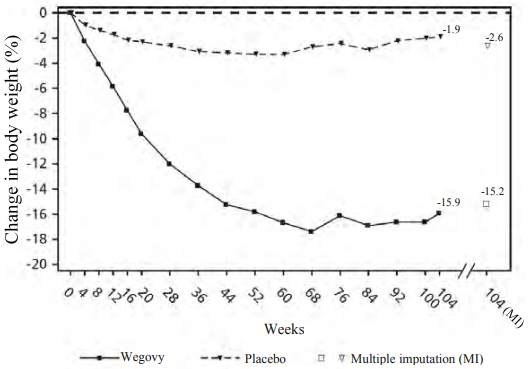
Treatment with semaglutide for 104 weeks resulted in a superior and clinically meaningful reduction in body weight compared to placebo. Mean body weight decreased from baseline through to week 68 with semaglutide after which a plateau was reached. With placebo, mean body weight decreased less, and a plateau was reached after approximately 20 weeks of treatment (see Table 8 and Figure 4). Patients treated with semaglutide achieved a mean change in body weight of -15.2%, with weight loss ≥5% achieved by 74.7%, ≥10% achieved by 59.2% and ≥15% achieved by 49.7% of these patients. Among patients with prediabetes at baseline, 80% and 37% achieved a normo-glycaemic status at end of treatment with semaglutide and placebo, respectively.
Table 8. STEP 5: Results at week 104:
| Wegovy | Placebo | |
|---|---|---|
| Full analysis set (N) | 152 | 152 |
| Body weight | ||
| Baseline (kg) | 105.6 | 106.5 |
| Change (%) from baseline1, 2 | -15.2 | -2.6 |
| Difference () from placebo1 [95 CI] | -12.6 [-15.3; -9.8]* | - |
| Change (kg) from baseline | -16.1 | -3.2 |
| Difference (kg) from placebo1 [95% CI] | -12.9 [-16.1; -9.8] | - |
| Patients () achieving weight loss ≥53 | 74.7* | 37.3 |
| Patients () achieving weight loss ≥103 | 59.2* | 16.8 |
| Patients () achieving weight loss ≥153 | 49.7* | 9.2 |
| Waist circumference (cm) | ||
| Baseline | 115.8 | 115.7 |
| Change from baseline1 | -14.4 | 5.2 |
| Difference from placebo1 [95% CI] | -9.2 [-12.2; -6.2]* | - |
| Systolic blood pressure (mmHg) | ||
| Baseline | 126 | 125 |
| Change from baseline1 | -5.7 | -1.6 |
| Difference from placebo1 [95% CI] | -4.2 [-7.3; -1.0]* | - |
* p<0.0001 (unadjusted 2-sided) for superiority.
1 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
2 During the trial, randomised treatment was permanently discontinued by 13.2% and 27.0% of patients randomised to semaglutide and placebo, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for body weight based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -16.7% and -0.6% for semaglutide and placebo respectively.
3 Estimated from binary regression model based on same imputation procedure as in primary analysis.
Figure 4. STEP 5: Mean change in body weight (%) from week 0 to week 104:
Observed values for patients completing each scheduled visit, and estimates with multiple imputations (MI) from retrieved dropouts
STEP 8: Semaglutide vs liraglutide
In a 68-week, randomised, open-label, pairwise placebo-controlled trial, 338 patients with obesity (BMI ≥30 kg/m²), or with overweight (BMI ≥27 to <30 kg/m²) and at least one weight-related comorbidity, were randomised to semaglutide once weekly, liraglutide 3 mg once daily or placebo. Semaglutide once weekly and liraglutide 3 mg were open-label, but each active treatment group was double-blinded against placebo administered at the same dosing frequency. All patients were on a reduced-calorie diet and increased physical activity throughout the trial. At baseline, patients had a mean BMI of 37.5 kg/m², a mean body weight of 104.5 kg.
Treatment with semaglutide once weekly for 68 weeks resulted in superior and clinically meaningful reduction in body weight compared to liraglutide. Mean body weight decreased from baseline through to week 68 with semaglutide. With liraglutide, mean body weight decreased less (see Table 9). 37.4% of the patients treated with semaglutide lost ≥20%, compared to 7.0% treated with liraglutide. Table 9 shows the results of the confirmatory endpoints ≥10%, ≥15% and ≥20% weight loss.
Table 9. STEP 8: Results of a 68-week trial comparing semaglutide with liraglutide:
| Wegovy | Liraglutide 3 mg | |
|---|---|---|
| Full analysis set (N) | 126 12 | 7 |
| Body weight | ||
| Baseline (kg) | 102.5 | 103.7 |
| Change (%) from baseline1,2 | -15.8 | -6.4 |
| Difference () from liraglutide1 [95 CI] | -9.4 [-12.0;-6.8]* | - |
| Change (kg) from baseline | -15.3 | -6.8 |
| Difference (kg) from liraglutide1 [95% CI] | -8.5 [-11.2;-5.7] | - |
| Patients () achieving weight loss ≥103 | 69.4* | 27.2 |
| Patients () achieving weight loss ≥153 | 54.0* | 13.4 |
| Patients () achieving weight loss ≥203 | 37.4* | 7.0 |
* p<0.005 (unadjusted 2-sided) for superiority.
1 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
2 During the trial, randomised treatment was permanently discontinued by 13.5% and 27.6% of patients randomised to semaglutide and liraglutide, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for body weight based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -16.7% and -6.7% for semaglutide and liraglutide respectively.
3 Estimated from binary regression model based on same imputation procedure as in primary analysis.
Effect on body composition
In a sub-study in STEP 1 (N=140), body composition was measured using dual energy X-ray absorptiometry (DEXA). The results of the DEXA assessment showed that treatment with semaglutide was accompanied by greater reduction in fat mass than in lean body mass leading to an improvement in body composition compared to placebo after 68 weeks. Furthermore, this reduction in total fat mass was accompanied by a reduction in visceral fat. These results suggest that most of the total weight loss was attributable to a reduction in fat tissue, including visceral fat.
Improvement in physical functioning
Semaglutide showed small improvements in physical functioning scores. Physical functioning was assessed using both the generic health-related quality of life questionnaire Short Form-36v2 Health Survey, Acute Version (SF-36) and the obesity-specific questionnaire Impact of Weight on Quality of Life Lite Clinical Trials Version (IWQOL-Lite-CT).
Cardiovascular evaluation
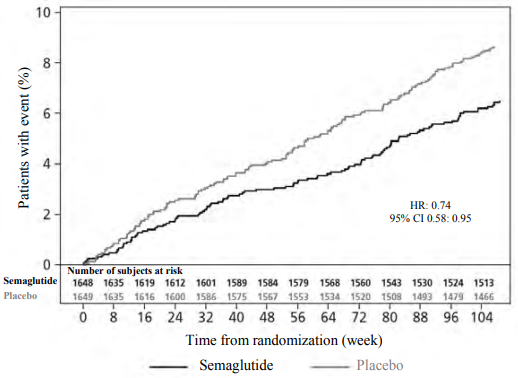
In the SUSTAIN 6 trial, 3,297 patients with insufficiently controlled type 2 diabetes and at high risk of cardiovascular events were randomised to semaglutide s.c. 0.5 mg or 1 mg once-weekly or placebo in addition to standard-of-care. The treatment duration was 104 weeks. The mean age was 65 years and the mean BMI was 33 kg/m².
The primary endpoint was the time from randomisation to first occurrence of a major adverse cardiovascular event (MACE): cardiovascular death, non-fatal myocardial infarction or non-fatal stroke. The total number of the MACE was 254, including 108 (6.6%) with semaglutide and 146 (8.9%) with placebo.
The cardiovascular safety of treatment with semaglutide 0.5 or 1 mg was confirmed as the hazard ratio (HR) for semaglutide vs. placebo was 0.74, [0.58, 0.95] [95% CI], driven by a decrease in the rate of non-fatal stroke and non-fatal myocardial infarction with no difference in cardiovascular death (see Figure 5).
Figure 5. Kaplan-Maier plot of time to first occurrence of the composite outcome: Cardiovascular death, non-fatal myocardial infarction or non-fatal stroke (SUSTAIN 6):
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Wegovy in one or more subsets of the paediatric population in the treatment of weight management (see section 4.2 for information on paediatric use).
STEP TEENS: Weight management in adolescent patients
In a 68-week double-blind trial 201 pubertal adolescents, ages 12 to <18 years, with obesity or overweight and at least one weight-related comorbidity were randomised 2:1 to semaglutide or placebo. All patients were on a reduced-calorie diet and increased physical activity throughout the trial.
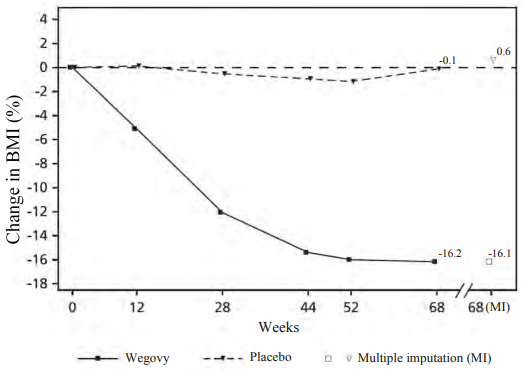
At end of treatment (week 68), the improvement in BMI with semaglutide was superior and clinically meaningful compared with placebo (see Table 10 and Figure 6). Furthermore, a higher proportion of patients achieved ≥5%, 10% and ≥15% weight loss with semaglutide compared with placebo (see Table 10).
Table 10. STEP TEENS: Results at week 68:
| Wegovy | Placebo | |
|---|---|---|
| Full analysis set (N) | 134 | 67 |
| BMI | ||
| Baseline (BMI) | 37.7 | 35.7 |
| Change (%) from baseline1,2 | -16.1 | 0.6 |
| Difference () from placebo1 [95 CI] | -16.7 [-20.3; -13.2]* | - |
| Baseline (BMI SDS) | 3.4 | 3.1 |
| Change from baseline in BMI SDS1 | -1.1 | -0.1 |
| Difference from placebo1 [95% CI] | -1.0 [-1.3; -0.8] | - |
| Body Weight | ||
| Baseline (kg) | 109.9 | 102.6 |
| Change (%) from baseline1 | -14.7 | 2.8 |
| Difference () from placebo1 [95 CI] | -17.4 [-21.1; -13.8] | - |
| Change (kg) from baseline1 | -15.3 | 2.4 |
| Difference (kg) from placebo1 [95% CI] | -17.7 [-21.8; -13.7] | - |
| Patients () achieving weight loss ≥53 | 72.5* | 17.7 |
| Patients () achieving weight loss ≥103 | 61.8 | 8.1 |
| Patients () achieving weight loss ≥153 | 53.4 | 4.8 |
| Waist circumference (cm) | ||
| Baseline | 111.9 | 107.3 |
| Change from baseline1 | -12.7 | -0.6 |
| Difference from placebo1 [95% CI] | -12.1 [-15.6; -8.7] | - |
| Systolic blood pressure (mmHg) | ||
| Baseline | 120 | 120 |
| Change from baseline1 | -2.7 | -0.8 |
| Difference from placebo1 [95% CI] | -1.9 [-5.0; 1.1] | - |
* p<0.0001 (unadjusted 2-sided) for superiority.
1 Estimated using an ANCOVA model using multiple imputation based on all data irrespective of discontinuation of randomised treatment or initiation of other anti-obesity medication or bariatric surgery.
2 During the trial, randomised treatment was permanently discontinued by 10.4% and 10.4% of patients randomised to semaglutide 2.4 mg and placebo, respectively. Assuming that all randomised patients stayed on treatment and did not receive additional anti-obesity therapies, the estimated changes from randomisation to week 68 for BMI based on a Mixed Model for Repeated Measures including all observations until first discontinuation were -17.9% and 0.6% for semaglutide 2.4 mg and placebo respectively
3 Estimated from logistic regression model based on same imputation procedure as in primary analysis.
Figure 6. STEP TEENS: Mean change in BMI (%) from baseline to week 68:
Observed values for patients completing each scheduled visit, and estimates with multiple imputations (MI) from retrieved dropouts
5.2. Pharmacokinetic properties
Compared to native GLP-1, semaglutide has a prolonged half-life of around 1 week making it suitable for once weekly subcutaneous administration. The principal mechanism of protraction is albumin binding, which results in decreased renal clearance and protection from metabolic degradation. Furthermore, semaglutide is stabilised against degradation by the DPP-4 enzyme.
Absorption
The average semaglutide steady state concentration following s.c. administration of the semaglutide maintenance dose was approximately 75 nmol/L in patients with overweight (BMI ≥27 kg/m² to <30 kg/m²) or obesity (BMI ≥30 kg/m²) based on data from phase 3a trials, where 90% of patients had average concentrations between 51 nmol/L and 110 nmol/L. The steady state exposure of semaglutide increased proportionally with doses from 0.25 mg up to 2.4 mg once weekly. Steady state exposure was stable with time as assessed up to week 68. Similar exposure was achieved with s.c. administration of semaglutide in the abdomen, thigh, or upper arm. The absolute bioavailability of semaglutide was 89%.
Distribution
The mean volume of distribution of semaglutide following s.c. administration in patients with overweight or obesity was approximately 12.4 L. Semaglutide is extensively bound to plasma albumin (>99%).
Metabolism/biotransformation
Prior to excretion, semaglutide is extensively metabolised through proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid side chain. The enzyme neutral endopeptidase (NEP) was identified as one of the active metabolic enzymes.
Elimination
The primary excretion routes of semaglutide-related material are via the urine and faeces. Approximately 3% of the absorbed dose was excreted in the urine as intact semaglutide. The clearance of semaglutide in patients with overweight (BMI ≥27 kg/m² to <30 kg/m²) or obesity (BMI ≥30 kg/m²) was approximately 0.05 L/h. With an elimination half-life of approximately 1 week, semaglutide will be present in the circulation for approximately 7 weeks after the last dose of 2.4 mg.
Special populations
Elderly
Age had no effect on the pharmacokinetics of semaglutide based on data from phase 3 trials including patients 18–86 years of age.
Gender, race and ethnicity
Gender, race (White, Black or African American, Asian) and ethnicity (Hispanic or Latino, nonHispanic or -Latino) had no effect on the pharmacokinetics of semaglutide based on data from phase 3a trials.
Body weight
Body weight had an effect on the exposure of semaglutide. Higher body weight was associated with lower exposure; a 20% difference in body weight between individuals will result in an approximate 18% difference in exposure. The 2.4 mg weekly dose of semaglutide provided adequate systemic exposures over the body weight range of 54.4−245.6 kg evaluated for exposure response in the clinical trials.
Renal impairment
Renal impairment did not impact the pharmacokinetics of semaglutide in a clinically relevant manner. This was shown with a single dose of 0.5 mg semaglutide for patients with different degrees of renal impairment (mild, moderate, severe or patients in dialysis) compared with patients with normal renal function. This was also shown for patients with overweight (BMI ≥27 kg/m² to <30 kg/m²) or obesity (BMI ≥30 kg/m²) and mild to moderate renal impairment based on data from phase 3a trials.
Hepatic impairment
Hepatic impairment did not have any impact on the exposure of semaglutide. The pharmacokinetics of semaglutide were evaluated in patients with different degrees of hepatic impairment (mild, moderate, severe) and compared with patients with normal hepatic function in a study with a single dose of 0.5 mg semaglutide.
Prediabetes and diabetes
Prediabetes and diabetes did not have any clinically relevant effect on the exposure of semaglutide based on data from phase 3 trials.
Immunogenicity
Development of anti-semaglutide antibodies when treated with semaglutide occurred infrequently (see section 4.8) and the response did not appear to influence semaglutide pharmacokinetics.
Paediatrics
Pharmacokinetic properties for semaglutide were assessed in a clinical trial for adolescent patients with obesity or overweight and at least one weight-related comorbidity ages 12 to <18 years (124 patients, body weight 61.6-211.9 kg). The semaglutide exposure in adolescents was similar to that in adults with obesity or overweight.
Safety and efficacy of semaglutide in children below 12 years of age have not been studied.
5.3. Preclinical safety data
Preclinical data reveal no special hazards for humans based on conventional studies of safety pharmacology, repeat-dose toxicity or genotoxicity.
Non-lethal thyroid C-cell tumours observed in rodents are a class effect for GLP-1 receptor agonists. In 2-year carcinogenicity studies in rats and mice, semaglutide caused thyroid C-cell tumours at clinically relevant exposures. No other treatment-related tumours were observed. The rodent C-cell tumours are caused by a non-genotoxic, specific GLP-1 receptor mediated mechanism to which rodents are particularly sensitive. The relevance for humans is considered to be low, but cannot be completely excluded.
In fertility studies in rats, semaglutide did not affect mating performance or male fertility. In female rats, an increase in oestrous cycle length and a small reduction in corpora lutea (ovulations) were observed at doses associated with maternal body weight loss.
In embryo-foetal development studies in rats, semaglutide caused embryotoxicity below clinically relevant exposures. Semaglutide caused marked reductions in maternal body weight and reductions in embryonic survival and growth. In foetuses, major skeletal and visceral malformations were observed, including effects on long bones, ribs, vertebrae, tail, blood vessels and brain ventricles. Mechanistic evaluations indicated that the embryotoxicity involved a GLP-1 receptor mediated impairment of the nutrient supply to the embryo across the rat yolk sac. Due to species differences in yolk sac anatomy and function, and due to lack of GLP-1 receptor expression in the yolk sac of non-human primates, this mechanism is considered unlikely to be of relevance to humans. However, a direct effect of semaglutide on the foetus cannot be excluded.
In developmental toxicity studies in rabbits and cynomolgus monkeys, increased pregnancy loss and slightly increased incidence of foetal abnormalities were observed at clinically relevant exposures. The findings coincided with marked maternal body weight loss of up to 16%. Whether these effects are related to the decreased maternal food consumption as a direct GLP-1 effect is unknown.
Postnatal growth and development were evaluated in cynomolgus monkeys. Infants were slightly smaller at delivery but recovered during the lactation period.
In juvenile rats, semaglutide caused delayed sexual maturation in both males and females. These delays had no impact upon fertility and reproductive capacity of either sex, or on the ability of the females to maintain pregnancy.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.