WILLFACT Powder and solvent for solution for injection Ref.[9434] Active ingredients: Von Willebrand factor
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2015 Publisher: LFB-BIOMEDICAMENTS, 3, avenue des Tropiques, BP 40305 LES ULIS, 91958 Courtabœuf Cedex, FRANCE
Therapeutic indications
Willfact is indicated in the prevention and treatment of haemorrhages or surgical bleeding in von Willebrand disease when desmopressin (DDAVP) treatment alone is ineffective or contraindicated.
Willfact should not be used in the treatment of haemophilia A.
Posology and method of administration
Treatment of von Willebrand disease should be supervised by a physician experienced in the treatment of haemostatic disorders.
Posology
Generally, 1 IU/kg of von Willebrand factor raises the circulating level of VWF:RCo by 0.02 IU/ml (2%).
Levels of VWF:RCo of >0.6 IU/ml (60%) and of FVIII:C of >0.4 IU/ml (40%) should be achieved.
Haemostasis cannot be ensured until FVIII coagulant activity (FVIII:C) has reached 0.4 IU/ml (40%). A single injection of von Willebrand factor will only lead to a maximum increase in FVIII:C after at least 6-12 hours. The single administration of von Willebrand factor cannot immediately correct the FVIII:C level. If the patient’s baseline plasma FVIII:C level is below this critical level, in all situations where a rapid correction of haemostasis should be achieved, such as the treatment of haemorrhage, severe trauma or emergency surgery, it is necessary to administer a factor VIII product with the first injection of von Willebrand factor, in order to achieve a haemostatic plasma level of FVIII:C.
However, if an immediate rise in FVIII:C is not necessary, for example in a planned operation, or if the baseline FVIII:C level is sufficient to ensure haemostasis, the physician may decide to omit the co-administration of FVIII at the first injection.
Start of treatment
The first dose of Willfact is 40 to 80 IU/kg for the treatment of haemorrhage or trauma, in conjunction with the required amount of factor VIII product, calculated according to the patient’s baseline plasma level of FVIII:C, in order to achieve an appropriate plasma level of FVIII:C, immediately before the intervention or as soon as possible after the onset of the bleeding episode or severe trauma. In case of surgery, it should be given 1 hour before the procedure.
An initial dose of 80 IU/kg of Willfact may be required, especially in patients with Type 3 von Willebrand disease where maintenance of adequate levels may require higher doses than in other types of VWD.
For elective surgery, treatment with Willfact should start 12-24 hours before surgery and should be repeated 1 hour before the procedure. In this case, co-administration of factor VIII product is not required since endogenous FVIII:C has usually reached the critical level of 0.4 IU/ml (40 %) before surgery. However, this should be confirmed in each patient.
Subsequent injections
If required, treatment should be continued with an appropriate dose of Willfact, with 40-80 IU/kg per day in 1 or 2 injections daily over one to several days. The dose and duration of the treatment depend on the clinical status of the patient, the type and severity of bleeding and both VWF:RCo and FVIII:C levels.
Long-term prophylaxis
Willfact can be administered as long-term prophylaxis in a dose which is determined individually for each patient. Willfact doses between 40 and 60 IU/kg, administered two to three times per week, reduce the number of haemorrhagic episodes.
Paediatric population
There is no data from a clinical study to characterise the response to use of Willfact in children less than 6 years of age.
The use of Willfact in children under 12 years of age is only documented in individual cases; the use of Willfact in patients previously untreated with von Willebrand factor is not documented in the clinical studies.
Method of administration
The product should be administered via the intravenous route at a maximum rate of 4 ml/minute.
For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
No case of overdose with Willfact has been reported.
Thromboembolic events may occur in case of major overdose.
Shelf life
3 years.
Chemical and physical in-use stability has been demonstrated for 24 hours at 25°C.
From a microbiological point of view, the product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user.
Special precautions for storage
Do not store above 25°C. Store in the original package in order to protect from light. Do not freeze.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
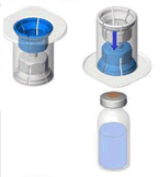
1 pack contains: powder in a vial (Type I glass) with a bromobutyl stopper, solvent in an injection vial (Type I glass) with a chlorobutyl stopper, and a transfer system.
Special precautions for disposal and other handling
Reconstitution:
The currently applicable guidelines for aseptic procedures must be followed. The transfer system is only used to reconstitute the drug, as described below. It is not intended in administering the drug to the patient.
- Bring the two vials (powder and solvent) to a temperature not above 25°C.
- Remove the protective cap from the solvent vial (water for injections) and from the powder vial.
- Disinfect the surface of each stopper.
- Remove the cap from the Mix2Vial device. Without removing the device from its packaging, attach the blue end of the Mix2Vial to the stopper of the solvent vial.
- Remove and discard the packaging. Take care not to touch the newly-exposed part of the device.
- Turn the solvent vial-device assembly over and attach to the powder vial using the transparent part of the device. The solvent will automatically transfer to the powder vial. Hold the assembly and gently swirl to completely dissolve the product.
- Now, holding the reconstituted product part in one hand and the solvent part in the other, unscrew the Mix2Vial device to separate the vials.
The powder generally dissolves instantaneously and should have dissolved in less than 10 minutes.
The solution should be clear or slightly opalescent, colourless or slightly yellowish.
Administration:
- Hold the vial of reconstituted product in vertical position while screwing a sterile syringe onto the Mix2Vial device. Then slowly draw the product up into the syringe.
- Once the product has been transferred to the syringe, firmly hold the syringe (with the piston pointing downward), unscrew the Mix2Vial device and replace it with an intravenous or butterfly needle.
- Expel the air from the syringe and insert into the vein after disinfecting the surface.
- Inject slowly by intravenous route immediately after reconstitution as a single dose at a maximum rate of 4 ml/minute.
Any unused product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.