WINREVAIR Solution for injection Ref.[109322] Active ingredients: Sotatercept
Source: FDA, National Drug Code (US) Revision Year: 2024
12. Clinical Pharmacology
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the study described below with the incidence of anti-drug antibodies in other studies, including those of WINREVAIR or of other sotatercept-csrk products. During the 24-week treatment period in STELLAR, 27% (44/163) of sotatercept-csrk-treated patients developed anti-sotatercept-csrk antibodies (ADA). Among the 44 ADA-positive patients, 12 (27%) tested positive for neutralizing antibodies against sotatercept-csrk. There were no identified clinical effects of anti-sotatercept-csrk antibodies on pharmacokinetics, pharmacodynamics, safety, or effectiveness of sotatercept-csrk over the treatment duration of 24 weeks at the recommended dosage.
12.1. Mechanism of Action
Sotatercept-csrk, a recombinant activin receptor type IIA-Fc (ActRIIA-Fc) fusion protein, is an activin signaling inhibitor that binds to activin A and other TGF-β superfamily ligands. As a result, sotatercept-csrk improves the balance between the pro-proliferative (ActRIIA/Smad2/3-mediated) and anti-proliferative (BMPRII/Smad1/5/8-mediated) signaling to modulate vascular proliferation. In rat models of PAH, a sotatercept-csrk analog reduced inflammation and inhibited proliferation of endothelial and smooth muscle cells in diseased vasculature. These cellular changes were associated with thinner vessel walls, partial reversal of right ventricular remodeling, and improved hemodynamics.
12.2. Pharmacodynamics
PVR
A statistically significantly greater decrease from baseline in PVR was observed in the WINREVAIR group compared to the placebo group in the Phase 3 STELLAR study. The median treatment difference in PVR between sotatercept-csrk and placebo was -235 dynes*sec/cm5 (95% CI: -288, -181; p<0.001). Sotatercept-csrk steady state exposure at 0.7 mg/kg dose was associated with near maximal reduction in PVR based on exposure-response analysis.
NT-proBNP
A statistically significantly greater decrease from baseline in NT-proBNP was observed in the WINREVAIR group compared to the placebo group in the Phase 3 STELLAR study. The median treatment difference in NT-proBNP between the sotatercept-csrk and placebo was -442 pg/mL (95% CI: -574, -310; p<0.001).
12.3. Pharmacokinetics
Following subcutaneous administration of 0.7 mg/kg WINREVAIR every three weeks to PAH patients, the steady state geometric mean (CV) area under the time concentration curve (AUC) is 172 mcg×d/mL (34.2), and peak concentration (Cmax) is 9.7 mcg/mL (30%). Sotatercept-csrk AUC and Cmax increased proportionally with dose. Steady state is achieved after approximately 15 weeks following initiation of multiple dosing. The accumulation ratio of sotatercept-csrk AUC is approximately 2.2.
Absorption
Following subcutaneous administration, the absolute bioavailability of sotatercept-csrk is approximately 66%. The sotatercept-csrk median time to peak drug concentration (Tmax) is approximately 7 days (range from 2 to 8 days) following multiple SC administration every 4 weeks.
Distribution
The population PK model estimated volume of distribution (CV) of sotatercept-csrk at steady state is approximately 5.3 L (27.3) in patients with PAH.
Elimination
The sotatercept-csrk effective half-life is approximately 24 days and its clearance is approximately 0.18 L/day.
Metabolism
Sotatercept-csrk is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in sotatercept-csrk pharmacokinetics (PK) were observed based on age (18 to 81 years of age), sex, race, mild to moderate (eGFR ranging from 30 to 89 mL/min) renal impairment (PAH patients), or end-stage kidney disease (eGFR <15 mL/min) with dialysis. Severe renal impairment (eGFR ranging from 15 to 30 mL/min) is not expected to impact the PK of sotatercept-csrk. Sotatercept-csrk is not dialyzable. The effect of hepatic impairment on the PK of sotatercept-csrk has not been studied.
Body Weight
The clearance (CL) and central volume of distribution (Vc) increase with increase in body weight. This effect is not clinically significant when sotatercept-csrk is administered using weight-based dosing as recommended.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies have been conducted with sotatercept-csrk. In a fertility and early embryonic development study in female rats, sotatercept-csrk was administered SC once weekly at doses of 5, 15, and 50 mg/kg beginning 2 weeks prior to mating and through gestation day 7. At doses ≥15 mg/kg (≥9 fold the MRHD, based on estimated AUC), pregnancy rates were decreased and there were increases in preimplantation and postimplantation loss and reductions in live litter size. Increased estrous cycle duration occurred at 50 mg/kg only (21-fold the MRHD, based on estimated AUC). In a fertility study in male rats, sotatercept-csrk was administered SC once weekly at doses of 0.3, 3, and 30 mg/kg for 13 weeks (beginning 10 weeks prior to mating). A subset of animals was examined after a 13-week recovery period. At ≥0.3 mg/kg (0.5-fold the MRHD, based on estimated AUC) there were non-reversible histologic changes in the efferent ducts, testes, and epididymides. Reversible decreases in functional fertility endpoints occurred at 30 mg/kg (20-fold the MRHD, based on estimated AUC).
14. Clinical Studies
14.1 Pulmonary Arterial Hypertension
The efficacy of WINREVAIR was evaluated in adult patients with PAH in the STELLAR trial (NCT04576988). STELLAR was a global, double-blind, placebo-controlled, multicenter, parallel-group clinical trial in which 323 patients with PAH (WHO Group 1 FC II or III) were randomized 1:1 to WINREVAIR (target dose 0.7 mg/kg) (n=163) or placebo (n=160) administered subcutaneously once every 3 weeks.
Participants were: 79% female; had a median age of 48 years (range: 18 to 82 years), and median body weight of 68 kg (range 38 to 141 kg); and 89% White/Caucasian, 2% Black/African American, 2% Asian, 0.3% American Indian or Alaska Native, 0.3% Native Hawaiian or Other Pacific Islander, 6% Missing/Other races. The most common PAH etiologies were idiopathic PAH (59%), heritable PAH (18%), and PAH associated with connective tissue diseases (CTD) (15%). STELLAR excluded patients with human immunodeficiency virus (HIV)-associated PAH, PAH associated with portal hypertension, schistosomiasis-associated PAH, and pulmonary veno occlusive disease. The mean time from PAH diagnosis to screening was 8.8 years. Most participants were receiving either three (61%) or two (35%) background drugs for PAH, and 40% were receiving prostacyclin infusions. Patients had a WHO FC II (49%) or III (51%) at baseline.
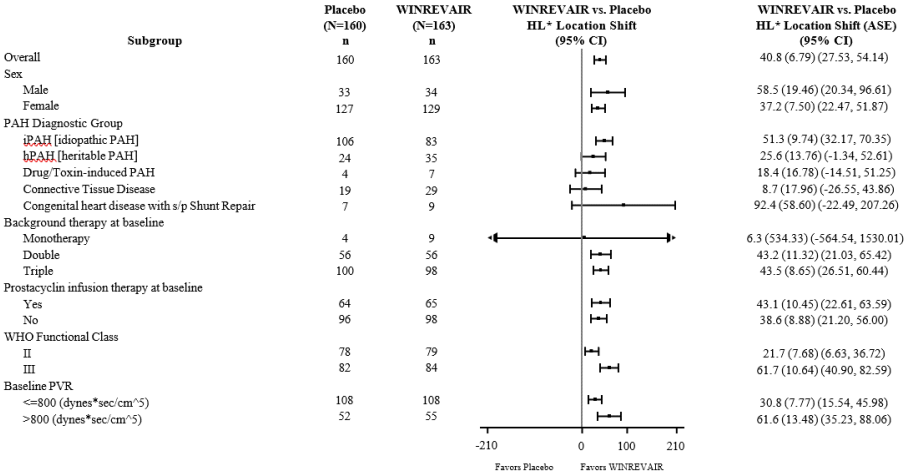
The primary efficacy endpoint was the change from baseline at Week 24 in 6-Minute Walk Distance (6 MWD). In the WINREVAIR group, the placebo-adjusted median increase in 6 MWD was 41 meters (95% CI: 28, 54; p<0.001). Figure 1 displays placebo-adjusted changes in 6 MWD at Week 24 in relevant subgroups.
Figure 1. Change from Baseline in 6-Minute Walk Distance (meters) at Week 24 in Subgroups:
* Hodges-Lehmann location shift from placebo estimate (median of all paired differences). ASE = asymptotic standard error.
Change from baseline in 6 MWD at Week 24 for subjects who died was imputed to -2000 meters to receive the worst rank. Change from baseline in 6 MWD at Week 24 for subjects who had missing data due to a non-fatal clinical worsening event was imputed to -1000 meters to receive the next-worst rank.
Treatment with WINREVAIR led to an improvement from baseline by at least 1 WHO FC at Week 24 in 29% of patients compared to 14% of patients treated with placebo (p<0.001).
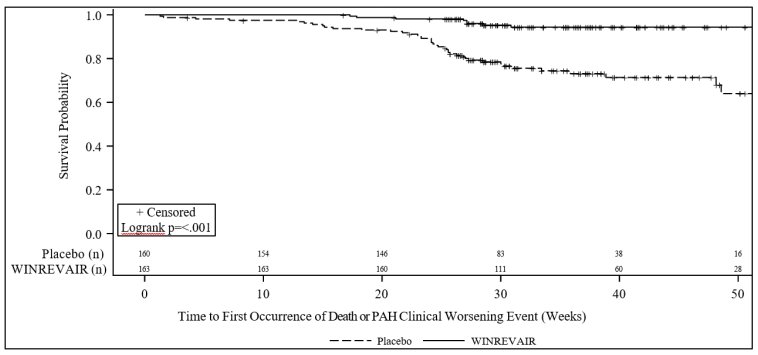
Treatment with WINREVAIR resulted in an 84% reduction in the occurrence of death from any cause or PAH clinical worsening events compared to placebo (see Table 4 and Figure 2). These outcomes were captured until the last patient completed the Week 24 visit (data up to the data cutoff; median duration of exposure 33.6 weeks).
Table 4. Death from Any Cause or PAH Clinical Worsening Events:
| Placebo (N=160) n (%) | WINREVAIR (N=163) n (%) | Hazard Ratio (95% CI) | |
|---|---|---|---|
| Number of subjects who experienced death or at least one clinical worsening event | 42 (26.3) | 9 (5.5) | 0.16 (0.08, 0.35) p<0.001 |
| Assessment of clinical worsening events* | |||
| Death | 7 (4.4) | 2 (1.2) | |
| Worsening-related listing for lung and/or heart transplant | 2 (1.3) | 1 (0.6) | |
| Need to initiate rescue therapy with an approved PAH therapy or the need to increase the dose of infusion prostacyclin by 10% or more | 17 (10.6) | 2 (1.2) | |
| Need for atrial septostomy† | 0 (0.0) | 0 (0.0) | |
| PAH-specific hospitalization (≥24 hours) | 8 (5.0) | 0 (0.0) | |
| Deterioration of PAH‡ | 15 (9.4) | 4 (2.5) | |
* A subject can have more than one assessment recorded for their clinical worsening.
† There were no events of atrial septostomy.
‡ Deterioration of PAH is defined by both of the following events occurring at any time, even if they began at different times, as compared to their baseline values: (a) Worsened WHO functional class (II to III, III to IV, II to IV, etc.); and (b) Decrease in 6 MWD by ≥15% (confirmed by two 6 MWTs at least 4 hours apart but no more than one week).
N = number of subjects in the category.
6 MWT = 6-Minute Walking Test
Figure 2. Time to Death from Any Cause or First Occurrence of PAH Clinical Worsening Event Kaplan-Meier Plot:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.