XACDURO Kit for injection Ref.[107381] Active ingredients: Sulbactam Sulbactam and Durlobactam
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
XACDURO (sulbactam for injection and durlobactam for injection) is an antibacterial co-packaged product containing sulbactam sodium, a penicillin derivative beta-lactam antibacterial and beta-lactamase inhibitor, and durlobactam sodium, a diazabicyclooctane beta-lactamase inhibitor, for intravenous administration.
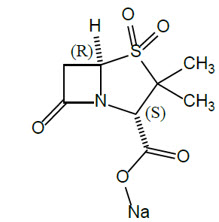
Sulbactam Sodium
Sulbactam sodium is a beta-lactam antibacterial and a beta-lactamase inhibitor. Chemically, sulbactam sodium is sodium penicillinate sulfone; sodium (2S, 5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo [3.2.0] heptane-2-carboxylate 4,4-dioxide. Sulbactam is a white to off-white crystalline powder that is freely soluble in water and its empirical formula is C8H10NNaO5S with a molecular weight of 255.22.
Figure 1. Chemical structure of sulbactam sodium:
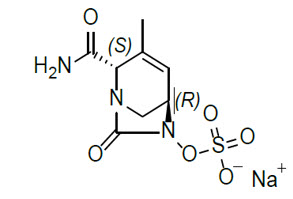
Durlobactam Sodium
Durlobactam is a beta-lactamase inhibitor antibacterial drug. Chemically, durlobactam sodium is sodium (2S,5R)-2-carbamoyl-3-methyl-7-oxo-1,6-diazabicyclo[3.2.1]oct-3-en-6-yl sulfate. Durlobactam sodium is the pure (2S,5R)-enantiomer of the trans diastereoisomer. It is a white to yellow amorphous powder that is freely soluble in water. The empirical formula is C8H10N3NaO6S and the molecular weight (sodium salt) is 299.23.
Figure 2. Chemical structure of durlobactam sodium:
XACDURO is supplied as a co-packaged kit containing three single-dose vials each containing sterile powder for reconstitution: one clear single-dose vial contains 1 g sulbactam (equivalent to 1.1 g sulbactam sodium) and two amber single-dose vials each contain 0.5 g durlobactam (equivalent to 0.54 g durlobactam sodium) together with sodium hydroxide and hydrochloric acid used for pH adjustment.
| Dosage Forms and Strengths |
|---|
|
XACDURO is a co-packaged kit containing the following two components as sterile powders for reconstitution:
|
| How Supplied |
|---|
|
XACDURO is a co-packaged product containing sulbactam for injection and durlobactam for injection. XACDURO is supplied as a kit (NDC 68547-111-10) containing the following single-dose vials each containing sterile powder for reconstitution:
Stoppers are not made with natural rubber latex. Distributed by: La Jolla Pharmaceutical Company, Waltham, MA 02451 |
Drugs
| Drug | Countries | |
|---|---|---|
| XACDURO | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.