XENPOZYME Powder for solution for infusion Ref.[50126] Active ingredients: Olipudase alfa
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Genzyme Europe B.V., Paasheuvelweg 25, 1105 BP Amsterdam, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other alimentary tract and metaolism products, Enzymes
ATC code: A16AB25
Mechanism of action
Olipudase alfa is a recombinant human acid sphingomyelinase that reduces sphingomyelin (SM) accumulation in organs of patients with Acid Sphingomyelinase Deficiency (ASMD).
Clinical efficacy and safety
The efficacy of Xenpozyme has been evaluated in 3 clinical studies (ASCEND study in adult patients, ASCEND-Peds study in paediatric patients and an extension study in adult and paediatric patients) involving a total of 61 patients with ASMD.
Clinical study in adult patients
The ASCEND study is a multicenter, randomised, double-blinded, placebo-controlled, repeat-dose phase II/III study in adult patients with ASMD type A/B and B. A total of 36 patients were randomised in a 1:1 ratio to receive either Xenpozyme or placebo. Treatment was administered in both groups as an intravenous infusion once every 2 weeks. Patients receiving Xenpozyme were up titrated from 0.1 mg/kg to a target dose of 3 mg/kg. The study was divided into 2 consecutive periods: a randomised placebo-controlled, double-blinded primary analysis period (PAP) which lasted to week 52, followed by an extension treatment period (ETP) for up to 4 years. Patients randomised to the placebo arm in the PAP crossed over to active treatment in the ETP to reach the targeted dose of 3 mg/kg, while patients in the original Xenpozyme arm continued treatment.
Patients enrolled in the study had a diffusion capacity of the lungs for carbon monoxide (DLco) ≤70% of the predicted normal value, a spleen volume ≥6 multiples of normal (MN) measured by magnetic resonance imaging (MRI) and scores ≥5 in splenomegaly related score (SRS). Overall, demographic and disease characteristics at baseline were similar between the two treatment groups. The median patient age was 30 years (range: 18-66 years). The mean (standard deviation, SD) age at ASMD diagnosis was 18 (18.4) years. At baseline, neurologic manifestations were seen in 9 out of 36 adult patients (25%) consistent with a clinical diagnosis of ASMD Type A/B. The remaining 27 patients had a clinical diagnosis consistent with ASMD Type B.
This study included 2 separate primary efficacy endpoints: the percentage change in DLco (in % predicted of normal) and spleen volume (in MN), as measured by MRI, from baseline to week 52. Secondary efficacy endpoints included the percentage change in liver volume (in MN) and platelet count from baseline to week 52. Pharmacodynamic parameters (ceramide and lyso-sphingomyelin [a deacylated form of SM] levels) were also assessed.
Improvements in mean percent change in % predicted DLco (p= 0.0004) and spleen volume (p< .0001) as well as in mean liver volume (p< .0001) and platelet count (p= 0.0185) were observed in the Xenpozyme group as compared to the placebo group during the 52-week primary analysis period. A significant improvement in mean percent change in % predicted DLco, spleen volume, liver volume and platelet count was noted at week 26 of treatment, the first post-dose endpoint assessment. The results from the PAP at week 52 are detailed in Table 6.
Table 6. Mean (SD) values for efficacy endpoints at baseline and least squares (LS) mean percentage change (SE) from baseline to week 52:
| Placebo (n=18) | Xenpozyme (n=18) | Difference [95% CI] | p value* | |
|---|---|---|---|---|
| Primary endpoints | ||||
| Mean % predicted DLco at baseline Percent change in % predicted DLco from baseline to week 52 | 48.5 (10.8) 3 (3.4) | 49.4 (11.0) 22 (3.3) | NA 19 (4.8) [9.3, 28.7] | NA 0.0004 |
| Mean spleen volume (MN) at baseline Percent change in spleen volume from baseline to week 52 | 11.2 (3.8) 0.5 (2.5) | 11.7 (4.9) -39.4 (2.4) | NA -39.9 (3.5) [-47.1, -32.8] | NA <0.0001 |
| Secondary endpoints | ||||
| Mean liver volume (MN) at baseline Percent change in liver volume from baseline to week 52 | 1.6 (0.5) -1.5 (2.5) | 1.4 (0.3) -28.1 (2.5) | NA -26.6 (3.6) [-33.9, -19.3] | NA <0.0001 |
| Mean platelet count (109/L) at baseline Percent change in platelet count from baseline to week 52 | 115.6 (36.3) 2.5 (4.2) | 107.2 (26.9) 16.8 (4.0) | NA +14.3 (5.8) [2.6, 26.1] | NA 0.0185 |
* Statistically significant after multiplicity adjustment
In addition, lyso-sphingomyelin, which is substantially elevated in plasma of ASMD patients, declined significantly, reflecting reduction of sphingomyelin content in tissue. The LS mean percentage change from baseline to week 52 (SE) in pre-infusion plasma lyso-sphingomyelin level was 77.7% (3.9) in the Xenpozyme treatment group compared to 5.0% (4.2) in the placebo group. The liver sphingomyelin content, as assessed by histopathology, decreased by 92.0% (SE: 8.1) from baseline to week 52 in the Xenpozyme treatment group (compared to +10.3% (SE: 7.8) in the placebo group).
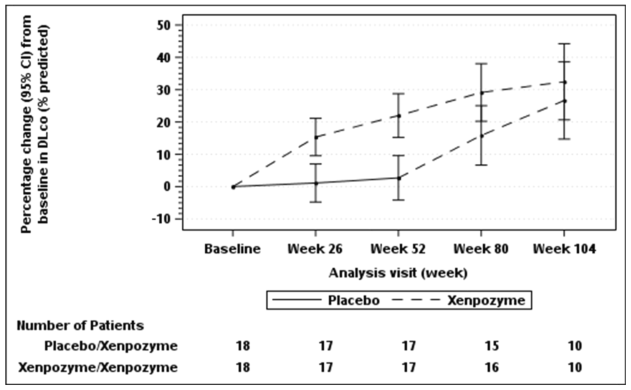
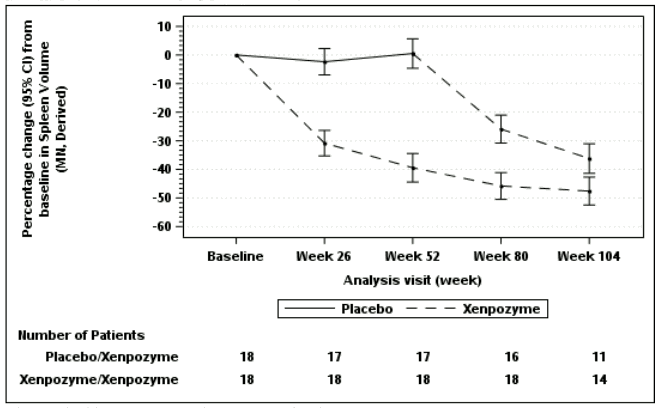
Seventeen of 18 patients previously receiving placebo and 18 of 18 patients previously treated with Xenpozyme for 52 weeks (PAP) started or continued treatment with Xenpozyme, respectively, for up to 4 years. Sustained effects of Xenpozyme on efficacy endpoints up to week 104 are presented in Figures 1 and 2 and Table 7.
Figure 1. Plot of the LS means (95%CI) of the percentage change in DLco (% predicted) from baseline to week 104 – mITT population:
The vertical bars represent the 95% CIs for the LS means.
The LS means and 95% CIs are based on a mixed model for repeated measures approach, using data up to week 104.
Patients in placebo/Xenpozyme group received placebo up to week 52 and switched to Xenpozyme thereafter.
Figure 2. Plot of the LS means (95%CI) of the percentage change in spleen volume (MN) from baseline to week 104 – mITT population:
The vertical bars represent the 95% CIs for the LS means.
The LS means and 95% CIs are based on a mixed model for repeated measures approach, using data up to week104.
Patients in placebo/Xenpozyme group received placebo up to week 52 and switched to Xenpozyme thereafter.
Table 7. LS mean percentage change (SE) from baseline to week 104 for liver volume (MN) and platelet count (109/L) in patients treated with Xenpozyme for 104 weeks:
| Previous olipudase alfa group | ||
|---|---|---|
| week 52 (ETP start) | week 104 | |
| N Percent change in liver volume (SD) | 17 -27.8 (2.5) | 14 -33.4 (2.2) |
| N Percent change in platelet count (SD) | 18 16.6 (4.0) | 13 24.9 (6.9) |
N: number of patients
Extension study in adult patients
Five adult patients who participated in an open-label ascending dose study in ASMD patients continued treatment in an open-label extension study and received Xenpozyme for up to >7 years. Sustained improvements in % predicted DLco, spleen and liver volumes and platelet count, compared to baseline, were noted in adult over the course of the study (see Table 8).
Table 8. Mean percentage change (SD) from baseline to month 78 of efficacy parameters:
| Month 78 (N=5) | |
|---|---|
| Percent change in % predicted DLco (SD) | 55.3% (48.1) |
| Percent change in spleen volume (SD) | -59.5% (4.7) |
| Percent change in liver volume (SD) | -43.7% (16.7) |
| Percent change in platelet count (SD) | 38.5% (14.7) |
N: number of patients
Paediatric population
The ASCEND-Peds study (Phase ½ clinical study) is a multi-center, open-label, repeated-dose study to evaluate the safety and tolerability of Xenpozyme administered for 64 weeks in paediatric patients aged <18 years with ASMD (type A/B and B). In addition, exploratory efficacy endpoints related to organomegaly, pulmonary and liver functions, and linear growth were evaluated at week 52.
A total of 20 patients (4 adolescents from 12 to <18 years old, 9 children from 6 to <12 years old, and 7 infants/children <6 years old) were up-titrated with Xenpozyme via a dose escalation regimen from 0.03 mg/kg to a target dose of 3 mg/kg. Treatment was administered as an intravenous infusion once every 2 weeks for up to 64 weeks. Patients enrolled in the study had a spleen volume ≥5 MN measured by MRI. Patients were distributed across all ages from 1.5 to 17.5 years old, with both sexes equally represented. The mean (SD) age at ASMD diagnosis was 2.5 (2.5) years. At baseline, neurologic manifestations were seen in 8 out of 20 paediatric patients (40%) consistent with a clinical diagnosis of ASMD Type A/B. The remaining 12 patients had a clinical diagnosis consistent with ASMD Type B.
Treatment with Xenpozyme resulted in improvements in mean percent change in % predicted DLco, spleen and liver volumes, platelet counts, and linear growth progression (as measured by Height Zscores) at week 52 as compared to baseline (see Table 9).
Table 9. LS Mean percentage change (SE) or change (SD) from baseline to week 52 (all age cohort) of efficacy parameters:
| Baseline value (n=20) | Week 52 (n=20) | |
|---|---|---|
| Mean % predicted DLco (SD) Percent change in % predicted DLco* 95% CI | 54.8 (14.2) | 71.7 (14.8) 32.9 (8.3) 13.4, 52.5 |
| Mean spleen volume (MN) (SD) Percent change in spleen volume (in MN) 95% CI | 19.0 (8.8) | 9.3 (3.9) -49.2 (2.0) -53.4, -45.0 |
| Mean liver volume (MN) (SD) Percent change in liver volume (in MN) 95% CI | 2.7 (0.7) | 1.5 (0.3) -40.6 (1.7) -44.1, -37.1 |
| Mean platelet count (109/L) (SD) Percent change in platelet count 95% CI | 137.7 (62.3) | 173.6 (60.5) 34.0 (7.6) 17.9, 50.1 |
| Mean height Z-scores (SD) Change in height Z-scores* 95% CI | -2.1 (0.8) | -1.6 (0.8) 0.6 (0.4) (0.38,0.73) |
* DLco was evaluated in 9 paediatric patients aged ≥5 years who were able to perform the test, change in height Z-score was evaluated in 19 paediatric patients.
In addition, LS mean pre-infusion plasma ceramide and lyso-sphingomyelin levels were reduced by 57% (SE: 5.1) and 87.2% (SE: 1.3), respectively, compared to baseline following 52 weeks of treatment.
The effects of Xenpozyme on spleen and liver volumes, platelets and height z-scores were seen across all paediatric age cohorts included in the study.
Extension study paediatric patients
Twenty paediatric patients who participated in ASCEND-Peds study continued treatment in an openlabel extension study and received Xenpozyme for up to >5 years. Sustained improvements in efficacy parameters (% predicted DLco, spleen and liver volumes, platelet counts, height Z-scores and bone age) were noted in paediatric patients over the course of the study up to month 48 (see Table 10).
Table 10. Mean percentage change or change (SD) from baseline to month 48 (all age cohort) of efficacy parameters:
| Month 48 | |
|---|---|
| N Percent change in % predicted DLco (SD) | 5 60.3 (58.5) |
| N Percent change in spleen volume (SD) | 7 -69.1 (4.1) |
| N Percent change in liver volume (SD) | 7 -55.4 (11.0) |
| N Percent change in platelet count (SD) | 5 35.8 (42.4) |
| N Change in height Z-scores (SD) | 5 2.3 (0.8) |
| N Change in bone age (months) (SD) | 7 18.5 (19.0) |
N: number of patients
The European Medicines Agency has deferred the obligation to submit the results of studies with Xenpozyme in one or more subsets of the paediatric population in the treatment of Acid Sphingomyelinase Deficiency (see 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
The pharmacokinetics (PK) of olipudase alfa were assessed in 49 adult ASMD patients from all clinical studies, receiving single or multiple administrations. At the dose of 3 mg/kg administered once every 2 weeks, the mean (percent coefficient of variation, CV%) maximum concentration (Cmax) and area under the concentration-time curve over a dosing interval (AUC0-τ) at steady state were 30.2 µg/mL (17%) and 607 µg.h/mL (20%), respectively.
Absorption
There is no absorption since Xenpozyme is administered intravenously.
Distribution
The estimated mean (CV%) volume of distribution of olipudase alfa is 13.1 L (18%).
Biotransformation
Olipudase alfa is a recombinant human enzyme and is expected to be eliminated via proteolytic degradation into small peptides and amino acids.
Elimination
The mean (CV%) clearance of olipudase alfa is 0.331 L/h (22%). The mean terminal half-life (t1/2) ranged from 31.9 to 37.6 hours.
Linearity/non-linearity
Olipudase alfa exhibited linear pharmacokinetics over the dose range of 0.03 to 3 mg/kg. Following a dose escalation regimen from 0.1 to the maintenance dose of 3 mg/kg administered once every 2 weeks, there was minimal accumulation in plasma levels of olipudase alfa.
Special populations
There were no clinically relevant differences in olipudase alfa pharmacokinetics based on gender.
Population pharmacokinetic analysis indicated that the exposure in Asian (n=2) and other race patients (n=2) were within the exposure ranges observed for Caucasian patients.
Elderly (≥65 years old)
Population pharmacokinetic analysis did not indicate a difference in exposure in elderly (only 2 patients between 65 and 75 years of age were included in clinical studies with Xenpozyme).
Paediatric
The PK of olipudase alfa were assessed in 20 paediatric patients including 4 adolescent patients, 9 child patients and 7 child/infant patients (Table 11). Olipudase alfa exposures were lower in paediatric patients compared to those in adult patients. However, these differences were not considered to be clinically relevant.
Table 11. Mean (CV%) of olipudase alfa PK parameters following administration of 3 mg/kg every 2 weeks in adolescent, child and child/infant patients with ASMD:
| Age Group | Age (year) | Cmax (µg/mL) | AUC0-τ (µg.h/mL) |
|---|---|---|---|
| Adolescent (n=4) | 12, <18 | 27.5 (8) | 529 (7) |
| Child (n=9) | 6, <12 | 24.0 (10) | 450 (15) |
| Child/Infant (n=7) | <6 | 22.8 (8) | 403 (11) |
Descriptive statistics represent the post hoc estimates of steady-state exposures using population PK analysis.
AUC0-τ: area under the plasma concentration versus time curve over a dosing interval; Cmax: maximum plasma concentration; n: total number of patients.
Hepatic impairment
Olipudase alfa is a recombinant protein and is expected to be eliminated by proteolytic degradation. Therefore, impaired liver function is not expected to affect the pharmacokinetics of olipudase alfa.
Renal impairment
Four patients (11.1%) with mild renal impairment (60 mL/min ≤ creatinine clearance <90 mL/min) were included in the ASCEND study. There were no clinically relevant differences in olipudase alfa pharmacokinetics in patients with mild renal impairment. The impact of moderate to severe renal impairment on the pharmacokinetics of olipudase alfa is not known. Olipudase alfa is not expected to be eliminated through renal excretion. Therefore, renal impairment is not expected to affect the pharmacokinetics of olipudase alfa.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on studies of safety pharmacology, single dose toxicity and repeated dose toxicity conducted in wild type animals (mice, rats, rabbits, dogs and monkeys) at dose levels 10 times above the Maximum recommended human dose (MRHD). Studies to evaluate the mutagenic and carcinogenic potential of olipudase alfa have not been performed.
In acid sphingomyelinase knockout (ASMKO) mice (a disease model for ASMD), mortality was observed following an administration of single doses of olipudase alfa ≥3.3 times higher than MRHD as an intravenous bolus injection. However, repeat dose studies show that administration of olipudase alfa via a dose escalation regimen did not result in compound-related mortality and reduced the severity of other toxicity findings up to the highest tested dose of 10 times the MRHD.
An increased incidence of exencephaly was observed when pregnant mice were treated daily with olipudase alfa at exposure levels comparable to the human exposure at the recommended maintenance therapeutic dose and frequency. This incidence was slightly higher than historical control data. The relevance of this observation for humans is unknown. The daily intravenous administration of olipudase alfa to pregnant rabbits did not result in fetal malformations or variations at exposures significantly exceeding the human exposure at the recommended maintenance therapeutic dose and frequency.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.