XEPLION Prolonged release suspension for injection Ref.[7473] Active ingredients: Paliperidone
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
Pharmacodynamic properties
Pharmacotherapeutic group: Psycholeptics, other antipsychotics
ATC code: N05AX13
Xeplion contains a racemic mixture of (+)- and (-)-paliperidone.
Mechanism of action
Paliperidone is a selective blocking agent of monoamine effects, whose pharmacological properties are different from that of traditional neuroleptics. Paliperidone binds strongly to serotonergic 5-HT2- and dopaminergic D2receptors. Paliperidone also blocks alpha-1-adrenergic receptors and slightly less, H1-histaminergic and alpha-2-adrenergic receptors. The pharmacological activity of the (+) and (-)-paliperidone enantiomers are qualitatively and quantitatively similar.
Paliperidone is not bound to cholinergic receptors. Even though paliperidone is a strong D2-antagonist, which is believed to relieve the positive symptoms of schizophrenia, it causes less catalepsy and decreases motor functions less than traditional neuroleptics. Dominating central serotonin antagonism may reduce the tendency of paliperidone to cause extrapyramidal side effects.
Clinical efficacy
Acute treatment of schizophrenia
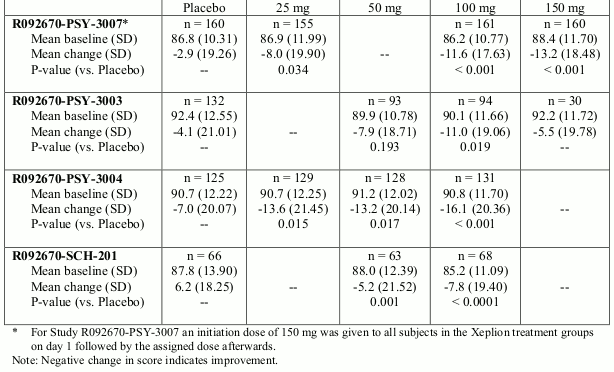
The efficacy of Xeplion in the acute treatment of schizophrenia was established in four short-term (one 9-week and three 13-week) double-blind, randomised, placebo-controlled, fixed-dose studies of acutely relapsed adult inpatients who met DSM-IV criteria for schizophrenia. The fixed doses of Xeplion in these studies were given on days 1, 8, and 36 in the 9-week study, and additionally on day 64 of the 13-week studies. No additional oral antipsychotic supplementation was needed during the acute treatment of schizophrenia with Xeplion. The primary efficacy endpoint was defined as a decrease in Positive and Negative Syndrome Scale (PANSS) total scores as shown in the table below.
The PANSS is a validated multi-item inventory composed of five factors to evaluate positive symptoms, negative symptoms, disorganised thoughts, uncontrolled hostility/excitement and anxiety/depression. Functioning was evaluated using the Personal and Social Performance (PSP) scale.
The PSP is a validated clinician rated scale that measures personal and social functioning in four domains: socially useful activities (work and study), personal and social relationships, self-care and disturbing and aggressive behaviours.
In a 13-week study (n=636) comparing three fixed doses of Xeplion (initial deltoid injection of 150 mg followed by 3 gluteal or deltoid doses of either 25 mg/4 weeks, 100 mg/4 weeks or 150 mg/4 weeks) to placebo, all three doses of Xeplion were superior to placebo in improving the PANSS total score. In this study, both the 100 mg/4 weeks and 150 mg/4 weeks, but not the 25 mg/4 weeks, treatment groups demonstrated statistical superiority to placebo for the PSP score. These results support efficacy across the entire duration of treatment and improvement in PANSS and was observed as early as day 4 with significant separation from placebo in the 25 mg and 150 mg Xeplion groups by day 8.
The results of the other studies yielded statistically significant results in favour of Xeplion, except for the 50 mg dose in one study (see table below).
Positive and Negative Syndrome Scale for Schizophrenia (PANSS) Total Score - Change From Baseline to End Point-LOCF for Studies R092670-SCH-201, R092670-PSY-3003, R092670-PSY-3004 and R092670-PSY-3007: Primary Efficacy Analysis Set:
Maintaining symptom control and delaying relapse of schizophrenia
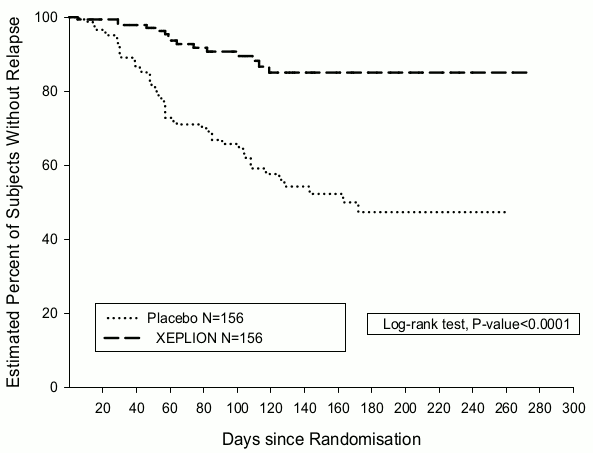
The efficacy of Xeplion in maintaining symptomatic control and delaying relapse of schizophrenia was established in a longer-term double-blind, placebo-controlled, flexible-dose study involving 849 non-elderly adult subjects who met DSM-IV criteria for schizophrenia. This study included a 33-week open-label acute treatment and stabilisation phase, a randomised, double-blind placebo-controlled phase to observe for relapse, and a 52-week open-label extension period. In this study, doses of Xeplion included 25, 50, 75, and 100 mg administered monthly; the 75 mg dose was allowed only in the 52-week open-label extension. Subjects initially received flexible doses (25-100 mg) of Xeplion during a 9-week transition period, followed by a 24-week maintenance period, where subjects were required to have a PANSS score of ≤75. Dosing adjustments were only allowed in the first 12 weeks of the maintenance period. A total of 410 stabilised patients were randomised to either Xeplion (median duration 171 days [range 1 day to 407 days]) or to placebo (median duration 105 days [range 8 days to 441 days]) until they experienced a relapse of schizophrenia symptoms in the variable length double-blind phase. The trial was stopped early for efficacy reasons as a significantly longer time to relapse (p<0.0001, Figure 1) was seen in patients treated with Xeplion compared to placebo (hazard ratio = 4.32; 95% CI: 2.4-7.7).
Figure 1. Kaplan-Meier Plot of Time to Relapse – Interim Analysis (Intent-to-Treat Interim Analysis Set):
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Xeplion in all subsets of the paediatric population in schizophrenia. See section 4.2 for information on paediatric use.
Pharmacokinetic properties
Absorption and distribution
Paliperidone palmitate is the palmitate ester prodrug of paliperidone. Due to its extremely low water solubility, paliperidone palmitate dissolves slowly after intramuscular injection before being hydrolysed to paliperidone and absorbed into the systemic circulation. Following a single intramuscular dose, the plasma concentrations of paliperidone gradually rise to reach maximum plasma concentrations at a median Tmax of 13 days. The release of the active substance starts as early as day 1 and lasts for at least 4 months.
Following intramuscular injection of single doses (25-150 mg) in the deltoid muscle, on average, a 28% higher Cmax was observed compared with injection in the gluteal muscle. The two initial deltoid intramuscular injections of 150 mg on day 1 and 100 mg on day 8 help attain therapeutic concentrations rapidly. The release profile and dosing regimen of Xeplion results in sustained therapeutic concentrations. The total exposure of paliperidone following Xeplion administration was dose-proportional over a 25-150 mg dose range, and less than dose-proportional for Cmax for doses exceeding 50 mg. The mean steady-state peak:trough ratio for a Xeplion dose of 100 mg was 1.8 following gluteal administration and 2.2 following deltoid administration. The median apparent half-life of paliperidone following Xeplion administration over the dose range of 25-150 mg ranged from 25-49 days.
The absolute bioavailability of paliperidone palmitate following Xeplion administration is 100%.
Following administration of paliperidone palmitate the (+) and (-) enantiomers of paliperidone interconvert, reaching an AUC (+) to (-) ratio of approximately 1.6-1.8.
The plasma protein binding of racemic paliperidone is 74%.
Biotransformation and elimination
One week following administration of a single oral dose of 1 mg immediate-release 14C-paliperidone, 59% of the dose was excreted unchanged into urine, indicating that paliperidone is not extensively metabolised in the liver. Approximately 80% of the administered radioactivity was recovered in urine and 11% in the faeces. Four metabolic pathways have been identified in vivo, none of which accounted for more than 6.5% of the dose: dealkylation, hydroxylation, dehydrogenation, and benzisoxazole scission. Although in vitro studies suggested a role for CYP2D6 and CYP3A4 in the metabolism of paliperidone, there is no evidence in vivo that these isozymes play a significant role in the metabolism of paliperidone. Population pharmacokinetics analyses indicated no discernible difference on the apparent clearance of paliperidone after administration of oral paliperidone between extensive metabolisers and poor metabolisers of CYP2D6 substrates. In vitro studies in human liver microsomes showed that paliperidone does not substantially inhibit the metabolism of medicinal products metabolised by cytochrome P450 isozymes, including CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5.
In vitro studies have shown that paliperidone is a P-gp substrate and a weak inhibitor of P-gp at high concentrations. No in vivo data are available and the clinical relevance is unknown.
Long acting paliperidone palmitate injection versus oral prolonged release paliperidone
Xeplion is designed to deliver paliperidone over a monthly period while prolonged release oral paliperidone is administered on a daily basis. The initiation regimen for Xeplion (150 mg/100 mg in the deltoid muscle on day 1/day 8) was designed to rapidly attain steady-state paliperidone concentrations when initiating therapy without the use of oral supplementation.
In general, overall initiation plasma levels with Xeplion were within the exposure range observed with 6-12 mg prolonged release oral paliperidone. The use of the Xeplion initiation regimen allowed patients to stay in this exposure window of 6-12 mg prolonged release oral paliperidone even on trough pre-dose days (day 8 and day 36). Because of the difference in median pharmacokinetic profiles between the two medicinal products, caution should be exercised when making a direct comparison of their pharmacokinetic properties.
Hepatic impairment
Paliperidone is not extensively metabolised in the liver. Although Xeplion was not studied on patients with hepatic impairment, no dose adjustment is required in patients with mild or moderate hepatic impairment. In a study with oral paliperidone in subjects with moderate hepatic impairment (Child-Pugh class B), the plasma concentrations of free paliperidone were similar to those of healthy subjects. Paliperidone has not been studied in patients with severe hepatic impairment.
Renal impairment
The disposition of a single oral dose paliperidone 3 mg prolonged release tablet was studied in subjects with varying degrees of renal function. Elimination of paliperidone decreased with decreasing estimated creatinine clearance. Total clearance of paliperidone was reduced in subjects with impaired renal function by 32% on average in mild (CrCl = 50 to <80 mL/min), 64% in moderate (CrCl = 30 to <50 mL/min), and 71% in severe (CrCl = 10 to <30 mL/min) renal impairment, corresponding to an average increase in exposure (AUCinf) of 1.5, 2.6, and 4.8 fold, respectively, compared to healthy subjects. Based on a limited number of observations with Xeplion in subjects with mild renal impairment and pharmacokinetic simulations, a reduced dose is recommended (see section 4.2).
Elderly
Population pharmacokinetics analysis showed no evidence of age related pharmacokinetics differences.
Body mass index (BMI)/body weight
Pharmacokinetic studies with paliperidone palmitate have shown somewhat lower (10-20%) plasma concentrations of paliperidone in patients who are overweight or obese in comparison with normal weight patients (see section 4.2).
Race
Population pharmacokinetics analysis of data from studies with oral paliperidone revealed no evidence of race-related differences in the pharmacokinetics of paliperidone following Xeplion administration.
Gender
No clinically significant differences were observed between men and women.
Smoking status
Based on in vitro studies utilising human liver enzymes, paliperidone is not a substrate for CYP1A2; smoking should, therefore, not have an effect on the pharmacokinetics of paliperidone. Effect of smoking on the pharmacokinetics of paliperidone was not studied with Xeplion. A population pharmacokinetic analysis based on data with oral paliperidone prolonged release tablets showed a slightly lower exposure to paliperidone in smokers compared with non-smokers. The difference is unlikely to be of clinical relevance.
Preclinical safety data
Repeat-dose toxicity studies of intramuscularly injected paliperidone palmitate (the 1-month formulation) and orally administered paliperidone in rat and dog showed mainly pharmacological effects, such as sedation and prolactin-mediated effects on mammary glands and genitals. In animals treated with paliperidone palmitate an inflammatory reaction was seen at the intramuscular injection site. Occasionally abscess formation occurred.
In rat reproduction studies with oral risperidone, which is extensively converted to paliperidone in rats and humans, adverse effects were seen on the birth weight and survival of the offspring. No embryotoxicity or malformations were observed following intramuscular administration of paliperidone palmitate to pregnant rats up to the highest dose (160 mg/kg/day) corresponding to 4.1 times the exposure level in humans at the maximum recommended dose of 150 mg. Other dopamine antagonists, when administered to pregnant animals, have caused negative effects on learning and motor development in the offspring.
Paliperidone palmitate and paliperidone were not genotoxic. In oral carcinogenicity studies of risperidone in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and mammary gland adenomas (both species) were seen. The carcinogenic potential of intramuscularly injected paliperidone palmitate was assessed in rats. There was a statistically significant increase in mammary gland adenocarcinomas in female rats at 10, 30 and 60 mg/kg/month. Male rats showed a statistically significant increase in mammary gland adenomas and carcinomas at 30 and 60 mg/kg/month which is 1.2 and 2.2 times the exposure level at the maximum recommended human 150 mg dose. These tumours can be related to prolonged dopamine D2 antagonism and hyperprolactinemia. The relevance of these tumour findings in rodents in terms of human risk is unknown.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.