XPOVIO Film-coated tablet Ref.[10395] Active ingredients: Selinexor
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
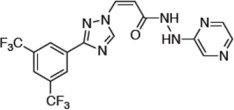
Selinexor is a nuclear export inhibitor. Selinexor is (2Z)3{3-[3,5-bis(trifluoromethyl)phenyl]-1H-1,2,4-triazol-1-yl}N'(pyrazin-2-yl)prop-2-enehydrazide. It is a white to off-white powder and has the molecular formula C17H11F6N7O and a molecular mass of 443.31 g/mol. The molecular structure is shown below:
Each XPOVIO (selinexor) tablet contains 20 mg of selinexor as the active ingredient. XPOVIO tablets are blue, round, bi-convex, film-coated tablets with “K20” debossed on one side and nothing on the other side. The inactive ingredients are colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, Opadry 200 clear, Opadry II blue, povidone K30, and sodium lauryl sulfate.
| Dosage Forms and Strengths |
|---|
|
Tablets: 20 mg, blue, round, bi-convex, film-coated tablets with “K20” debossed on one side and nothing on the other side. |
| How Supplied | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
XPOVIO (selinexor) are blue, round, bi-convex, and film-coated 20 mg tablets with “K20” debossed on one side and nothing on the other side. Tablets are packaged in a child-resistant blister pack. Four blister packs are supplied per carton. The following seven dose presentations are available:
Manufactured for and marketed by: Karyopharm Therapeutics Inc., 85 Wells Avenue, Newton, MA, 02459. |
Drugs
| Drug | Countries | |
|---|---|---|
| XPOVIO | Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.