YESCARTA Dispersion for infusion Ref.[8759] Active ingredients: Axicabtagene ciloleucel
Source: European Medicines Agency (EU) Revision Year: 2024 Publisher: Kite Pharma EU B.V., Tufsteen 1, 2132 NT Hoofddorp, The Netherlands
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, other antineoplastic agents, antineoplastic cell and gene therapy
ATC code: L01XL03
Mechanism of action
Yescarta, an engineered autologous T-cell immunotherapy product, binds to CD19 expressing cancer cells and normal B-cells. Following anti-CD19 CAR T-cell engagement with CD19 expressing target cells, the CD28 and CD3-zeta co-stimulatory domains activate downstream signalling cascades that lead to T-cell activation, proliferation, acquisition of effector functions, and secretion of inflammatory cytokines and chemokines. This sequence of events leads to apoptosis and necrosis of CD19-expressing target cells.
Pharmacodynamic effects
After Yescarta infusion, pharmacodynamic responses were evaluated by measuring transient elevation of cytokines, chemokines, and other molecules in blood over a 4-week interval. Levels of cytokines and chemokines such as IL-6, IL-8, IL-10, IL-15, TNF-α, IFN-γ, and IL2Rα were analyzed. Peak elevation was observed within the first 14 days after infusion, and levels generally returned to baseline within 28 days.
Analyses performed to identify associations between cytokine levels and incidence of CRS or neurologic events showed that higher post-infusion levels (peak and AUC at 1 month) of multiple immune-modulatory and pro-inflammatory analytes were associated with Grade 3 or higher neurologic adverse reactions and Grade 3 or higher CRS in ZUMA-1, ZUMA-7 and ZUMA-5.
Due to the on-target, off-tumour effect of Yescarta, a period of B-cell aplasia is expected following treatment.
Among 73 patients in ZUMA-1 with evaluable samples at baseline, 40% had detectable B-cells; the B-cell aplasia observed in the majority of patients at baseline was attributed to prior therapies. Following Yescarta treatment, the proportion of patients with detectable B-cells decreased: 20% had detectable B-cells at Month 3, and 22% had detectable B-cells at Month 6. The initiation of B-cell recovery was first noted at Month 9, when 56% of patients had detectable B-cells. This trend of B-cell recovery continued over time, as 64% of patients had detectable B-cells at Month 18, and 77% of patients had detectable B-cells at Month 24. Among 141 patients in ZUMA-7 with evaluable samples at baseline, 57% had detectable B-cells. Following Yescarta treatment, the proportion of patients with detectable B-cells decreased: 38% had detectable B-cells at Month 3, and 41% had detectable B-cells at Month 6. The initiation of B-cell recovery was apparent at Month 9, when 58% had detectable B-cells. This trend of B-cell recovery continued over time, as 64% of patients had detectable B-cells at Month 18 and 84% of patients had detectable B-cells at Month 24. Among 113 FL patients with evaluable samples at baseline in ZUMA-5, 75% of patients had detectable B-cells. Following Yescarta treatment, the proportion of patients with detectable B-cells decreased: 40% of patients had detectable B-cells at Month 3. B-cell recovery was observed over time, with 61% of patients having detectable B-cells at Month 24. Patients were not required to be followed after they progressed; thus, the majority of patients with evaluable samples were responders.
Clinical efficacy and safety
Relapsed or refractory DLBCL, PMBCL and DLBCL arising from follicular lymphoma after two or more lines of systemic therapy (ZUMA-1)
A total of 108 patients were treated with Yescarta in a phase ½ open-label, multicentre, single-arm study in patients with r/r aggressive B-cell non-Hodgkin lymphoma (NHL). Efficacy was based on 101 patients in phase 2, including histologically confirmed DLBCL (N=77), PMBCL (N=8), or DLBCL arising from follicular lymphoma, (N=16) based on the 2008 WHO-classification. DLBCL in ZUMA-1 included patients with DLBCL not otherwise specified (NOS), other DLBCL subtypes, and HGBL based on the 2016 WHO-classification. Forty-seven patients were evaluable for MYC, BCL-2, and BCL-6 status. Thirty were found to have double expressor DLBCL (overexpression of both MYC and BCL-2 protein); 5 were found to have HGBL with MYC, BCL-2 or BCL-6 gene rearrangement (double- and triple-hit); and 2 were found to have HGBL not otherwise specified. Sixty-six patients were evaluable for cell-of-origin classifications (germinal center B-cell type [GCB] or activated B-cell type [ABC]). Of these, 49 patients had GCB-type and 17 patients had ABC-type.
Eligible patients were ≥18 years of age with refractory disease defined as progressive disease (PD) or stable disease (SD) as best response to last line of therapy, or disease progression within 12 months after autologous stem cell transplant (ASCT). Patients who were refractory to chemotherapy or who relapsed after two or more lines of systemic therapy were generally ineligible for haematopoietic stem cell transplantation. Patients must have received at least prior anti-CD20 antibody therapy and an anthracycline containing regimen. Patients with CNS lymphoma, a history of allogeneic stem cell transplantation (SCT) or prior anti-CD19 CAR or other genetically modified T-cell therapy were excluded. Patients with a history of CNS disorders (such as seizures or cerebrovascular ischemia), cardiac ejection fraction of less than 50% or room air oxygen saturation of less than 92%, or autoimmune disease requiring systemic immunosuppression were ineligible. The median duration of follow-up was 63.1 months (still ongoing). A summary of the patient demographics is provided in Table 4.
Table 4. Summary of demographics for ZUMA-1 phase 2 (12 month analysis):
| Category | All leukapheresed (ITT) Cohort 1 + 2 (N=111) | All treated (mITT) Cohort 1 + 2 (N=101) |
|---|---|---|
| Age (years) | ||
| Median (min, max) | 58 (23, 76) | 58 (23, 76) |
| ≥65 | 23% | 24% |
| Male gender | 69% | 67% |
| Race | ||
| White | 85% | 86% |
| Asian | 4% | 3% |
| Black | 4% | 4% |
| ECOG status | ||
| ECOG 0 | 41% | 42% |
| ECOG 1 | 59% | 58% |
| Median number of prior therapies (min, max) | 3 (1, 10) | 3 (1, 10) |
| Patients with refractory disease to ≥2 prior lines of therapy | 77% | 76% |
| Patients relapsed within 1 year of ASCT | 20% | 21% |
| Patients with International Prognostic Index ¾ | 46% | 46% |
| Patients with disease stage III/IV | 85% | 85% |
ASCT, autologous stem cell transplant; ECOG, Eastern Cooperative Oncology Group; ITT, intention-to-treat; mITT, modified intention-to-treat.
Yescarta was administered as a single infusion at a target dose of 2 × 106 anti-CD19 CAR T cells/kg after lymphodepleting chemotherapy regimen of 500 mg/m² intravenous cyclophosphamide and 30 mg/m² intravenous fludarabine on the 5th, 4th, and 3rd day before Yescarta. Bridging chemotherapy between leukapheresis and lymphodepleting chemotherapy was not permitted. All patients were hospitalized for observation for a minimum of 7 days after Yescarta infusion.
Of 111 patients who underwent leukapheresis, 101 received Yescarta. Nine patients were not treated, primarily due to progressive disease or serious adverse events after enrolment and prior to cell delivery. One out of 111 patients did not receive the product due to manufacturing failure. The median time from leukapheresis to product delivery was 17 days (range: 14 to 51 days), and the median time from leukapheresis to infusion was 24 days (range: 16 to 73 days). The median dose was 2.0 × 106 anti-CD19 CAR T cells/kg. ITT was defined as all patients who underwent leukapheresis; mITT was defined as all patients who received Yescarta.
The primary endpoint was objective response rate (ORR). Secondary endpoints included duration of response (DOR), overall survival (OS), and severity of adverse events. The ORR was prespecified to be tested in the first 92 treated patients and was significantly higher than the prespecified rate of 20% (P<0.0001).
In the primary analysis, based on the mITT population (minimum follow-up of 6 months) the ORR was 72% and the complete response (CR) rate was 51%, as determined by an independent review committee. In the 12 month follow-up analysis (Table 5), the ORR was 72% and the CR rate was 51%. The median time to response was 1.0 months (range: 0.8 to 6.3 months). The DOR was longer in patients who achieved CR, as compared to patients with a best response of partial response (PR). Of the 52 patients who achieved CR, 7 patients had SD and 9 had PR at their initial tumour assessment and converted to CR as late as 6.5 months. The ORR results within PMBCL and DLBCL arising from follicular lymphoma were both 88%. CR rates were 75% and 56%, respectively. Of the 111 patients in the ITT population, the ORR was 66% and the CR was 47%. Other outcomes were consistent with those of the mITT population.
In the 24-month follow-up analysis, based on the mITT population (results from an independent review committee), the ORR and the CR rate were 74% and 54%, respectively. The median time to response was 1.0 months (range: 0.8 to 12.2 months). The DOR was longer in patients who achieved CR compared to patients with a best response of PR (Table 5). Of the 55 patients who achieved CR, 7 patients had SD and 10 had PR at their initial tumour assessment and converted to CR as late as 12 months after Yescarta infusion. Median duration of response and median OS had not been reached (Table 5). In a 36-month analysis (median study follow-up of 39.1 months) the median OS was 25.8 months with 47 patients (47%*) still alive. In a 48-month analysis (median study follow-up of 51.1 months) the median OS was 25.8 months with 43 patients (44%*) still alive. In a 60-month analysis (median study follow-up of 63.1 months) the median overall survival was 25.8 months with 42 patients (43%*) still alive. *The Kaplan-Meier estimates of the 3-year, 4-year and 5-year OS rates were 47%, 44% and 43% respectively.
In the phase 1 part of ZUMA-1, 7 patients were treated. Five patients responded, including 4 CRs. At the 12-month follow-up analysis, 3 patients remained in CR 24 months after Yescarta infusion. At the 24-month follow-up analysis, these 3 patients remained in CR at 30 to 35 months after Yescarta infusion.
Table 5. Summary of efficacy results for ZUMA-1 phase 2:
| Category | All leukapheresed (ITT) Cohort 1 + 2 (N=111) | All treated (mITT) Cohort 1 + 2 (N=101) | ||
|---|---|---|---|---|
| 12-month analysis | 24-month analysis | 12-month analysis | 24-month analysis | |
| ORR (%) [95% CI] | 66 (56, 75) | 68 (58, 76) | 72 (62, 81) | 74 (65, 82) |
| CR (%) | 47 | 50 | 51 | 54 |
| DORa, median (range) in months | 14.0 (0.0, 17.3) | NE (0.0, 29.5) | 14.0 (0.0, 17.3) | NE (0.0, 29.5) |
| DORa, CR, median (range) in months | NE (0.4, 17.3) | NE (0.4, 29.5) | NE (0.4, 17.3) | NE (0.4, 29.5) |
| OS, median (months) [95% CI] | 17.4 (11.6, NE) | 17.4 (11.6, NE) | NE (12.8, NE) | NE (12.8, NE) |
| 6 month OS (%) [95% CI] | 81.1 (72.5, 87.2) | 81.1 (72.5, 87.2) | 79.2 (69.9, 85.9) | 79.2 (69.9, 85.9) |
| 9 month OS (%) [95% CI] | 69.4 (59.9, 77.0) | 69.4 (59.9, 77.0) | 69.3 (59.3, 77.3) | 69.3 (59.3, 77.3) |
| 12 month OS (%) [95% CI] | 59.3 (49.6, 67.8) | 59.5 (49.7, 67.9) | 60.4 (50.2, 69.2) | 60.4 (50.2, 69.2) |
| 24 month OS (%) [95% CI] | Not applicable | 47.7 (38.2, 56.7) | Not applicable | 50.5 (40.4, 59.7) |
CI, confidence interval; CR, complete response; DOR, duration of response; ITT, intention-to-treat; mITT, modified intention-to-treat; NE= Not estimable (not reached); ORR, objective response rate; OS, overall survival.
a Duration of response was censored at the time of SCT for patients who received SCT while in response
Note: The 12-month analysis had a median follow-u p of 15.1 months. The 24-month analysis had a median follow-up of 27.1 months. Overall survival relates to the time from the leukapheresis date (ITT) or Yescarta infusion (mITT) to death from any cause.
SCHOLAR-1
A retrospective, patient-level, pooled analysis of outcomes in refractory aggressive NHL (N = 636) was conducted (Crump et al., 2017) to provide confirmation of the prespecified control response rate of 20% and historical context for interpreting the ZUMA-1 results. The analysis included patients who had not responded (SD or PD) to their last line of therapy or had relapsed within 12 months after ASCT. Response and survival after treatment with available standard-of-care therapy was evaluated. The ORR was 26% [95% CI (21, 31)] and the CR rate was 7% [95% CI (3, 15)], with a median OS of 6.3 months.
DLBCL and HGBL that relapses within 12 months from completion of, or is refractory to, first-line chemoimmunotherapy (ZUMA-7)
The efficacy and safety of Yescarta in adult patients with r/r large B-cell lymphoma (LBCL) was demonstrated in a Phase 3 randomised, open-label, multicenter study (ZUMA-7). Enrolled patients were predominantly diagnosed with DLBCL and HGBL disease subtypes based on the 2016 WHO-classification and all patients had received first-line rituximab and anthracycline-based chemotherapy. In total, 359 patients were randomised in a 1:1 ratio to receive a single infusion of Yescarta or to receive SOCT (defined as 2 to 3 cycles of standard chemoimmunotherapy [R-ICE, R-DHAP or R-DHAX, R-ESHAP, or R-GDP] followed by high-dose therapy [HDT] and ASCT in those with disease response). Randomisation was stratified by response to first-line therapy (primary refractory, vs relapse ≤6 months of first-line therapy vs relapse >6 and ≤12 months of first-line therapy) and second-line age-adjusted International Prognostic Index (IPI) (0 to 1 vs 2 to 3) as assessed at the time of screening. The study excluded prior HSCT, detectable cerebrospinal fluid malignant cells or brain metastases, Eastern Cooperative Oncology Group (ECOG) performance status of 2 or greater, and any history of central nervous system lymphoma. Patients with active or serious infections were excluded, however patients with simple urinary tract infection and uncomplicated bacterial pharyngitis were permitted if responding to active treatment.
Following lymphodepleting chemotherapy, Yescarta was administered as a single intravenous infusion at a target dose of 2 × 106 anti-CD19 CAR T cells/kg (maximum dose: 2 × 108 cells). The lymphodepleting regimen consisted of cyclophosphamide 500 mg/m² intravenously and fludarabine 30 mg/m² intravenously, both given on the 5th, 4th, and 3rd day before Yescarta. Nondisease modifying bridging therapy limited to corticosteroids, could be administered between leukapheresis and lymphodepleting chemotherapy for patients with high disease burden at screening.
In the overall study population, the median age was 59 years (range: 21 to 81 years); 66% were male, and 83% were white. Seventy-four percent of patients had primary refractory LBCL and 26% of patients had relapsed within 12 months of first-line therapy. Patients had a second-line age-adjusted IPI score of 0-1 (55%) or 2-3 (45%) and an ECOG performance status of 0 (54%) or 1 (46%).
Patients in the Yescarta and SOCT arms were categorized as DLBCL NOS/without further classification possible (126 patients and 120 patients, respectively); DLBCL arising from follicular lymphoma (19 patients and 27 patients, respectively); HGBL with MYC, BCL2, and/or BCL6 (double- and triple-hit) rearrangements (31 patients and 25 patients, respectively) or HGBL NOS, (1 patient in the SOCT arm); the remaining subjects were categorized under not confirmed, missing, or other.
Of the 180 patients randomised to receive Yescarta, 178 underwent leukapheresis and 170 were treated with Yescarta. Of the patients treated, 60 (33%) received bridging corticosteroid therapy. There were no manufacturing failures. Eight patients (4%) were not treated following leukapheresis, primarily due to progressive disease, serious adverse events, or death. The median time from leukapheresis to product release was 13 days (range: 10 to 24 days), and leukapheresis to Yescarta infusion was 26 days (range: 16 to 52 days). The median dose was 2.0 × 106 anti-CD19 CAR T cells/kg. All 170 patients who received Yescarta were monitored at a healthcare facility for a minimum of 7 days. Of the 179 patients randomised to receive SOCT, 64 patients (36%) received HDT-ASCT.
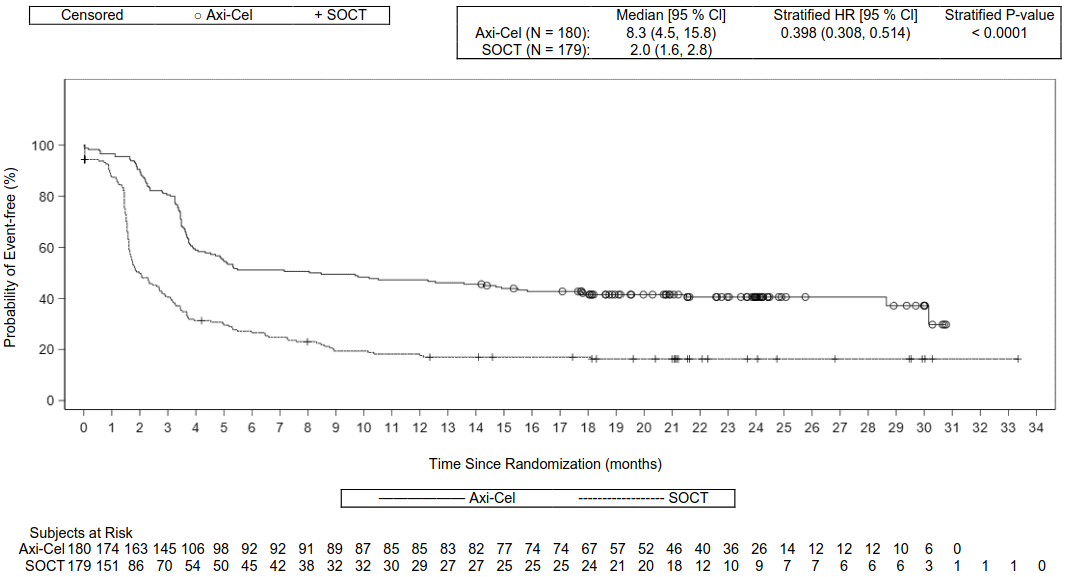
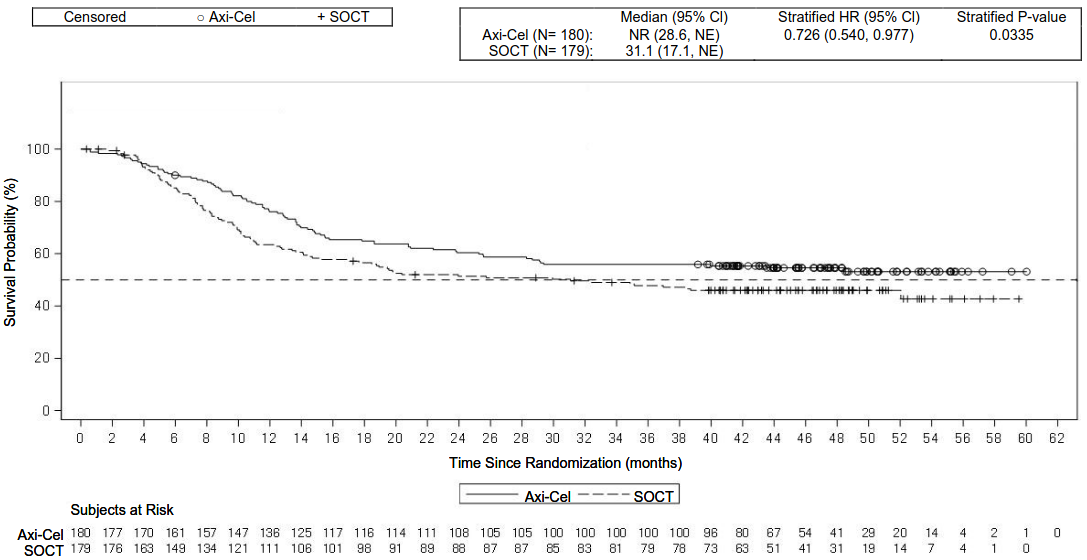
The primary endpoint was event-free survival (EFS) as determined by blinded central review. Key secondary endpoints were ORR and OS. The summary of efficacy results in the overall population is shown in Table 6 and the Kaplan-Meier curves for EFS and OS are shown in Figure 1 and Figure 2, respectively. The 24-month EFS was 40.5% [95% CI: 33.2, 47.7] in the Yescarta arm and 16.3% [95% CI: 11.1, 22.2] in the SOCT arm. At the time of the primary EFS analysis, the median progression free survival (PFS) per central assessment in the Yescarta arm was 14.7 months (95% CI: 5.4, NE) compared with 3.7 months (95% CI: 2.9, 5.3) in the SOCT arm (HR: 0.490 [95% CI: 0.368, 0.652]). The median study duration was 24.9 months at the time of the primary EFS analysis and 47.2 months at the time of the primary OS analysis. The primary analysis of OS was performed at the protocol- specified timepoint of 5 years from the first subject enrolled. A statistically significant improvement in OS in favour of Yescarta was demonstrated (see Table 6). The estimated 48-month OS rates were 54.6% in the Yescarta arm and 46.0% in the SOCT arm. Fifty-seven percent of patients received cellular immunotherapy after no response to or relapse after randomisation to SOCT.
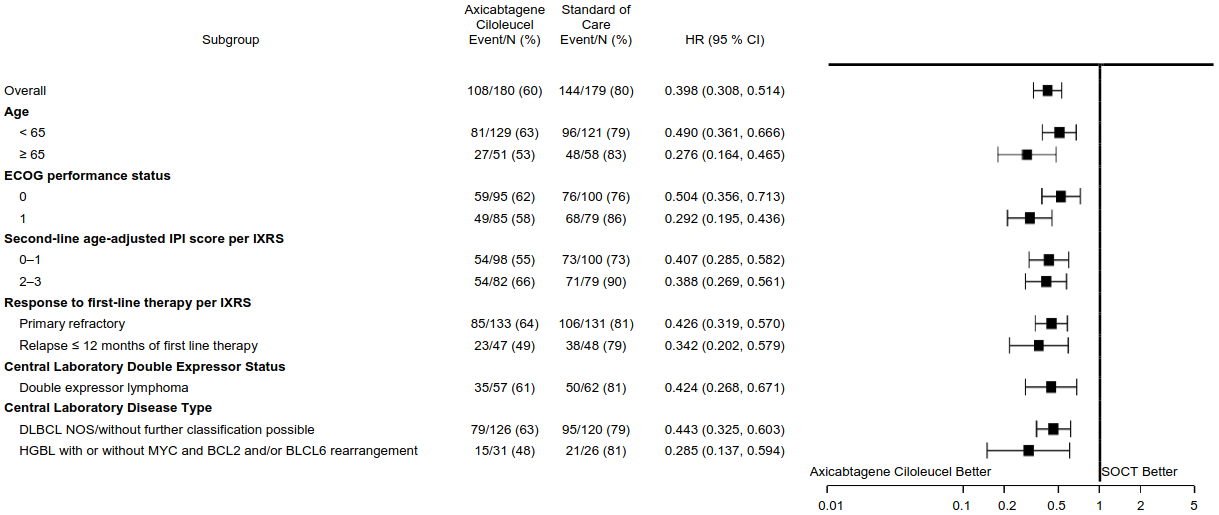
Consistent efficacy favouring Yescarta was generally observed across selected subgroups including response to first-line therapy, second-line age-adjusted IPI score, ECOG performance status, age, double expressor lymphoma status and HGBL disease subtype (see Figure 3). Among patients with HGBL per central laboratory, Yescarta demonstrated an improvement in EFS compared to SOCT (HR: 0.285 [95% CI: 0.137, 0.594]). The ORR was 81% (95% CI: 62.5%, 92.5%) and CR rate was 68% (95% CI: 48.6%, 83.3%) in patients treated with Yescarta compared with 42% (95% CI: 23.4%, 63.1%) and 23% (95% CI: 9.0%, 43.6%) respectively in the SOCT arm. The OS HR for Yescarta versus SOCT was 0.735 [95% CI: 0.338, 1.600] for patients with HGBL per central laboratory.
Table 6. Summary of Efficacy Results for ZUMA-7:
| Yescarta N=180 | Standard of Care Therapy N=179 | |
|---|---|---|
| EFSa | ||
| Number of events (%) | 108 (60) | 144 (80) |
| Median, months [95% CI]b | 8.3 [4.5, 15.8] | 2.0 [1.6, 2.8] |
| Stratified hazard ratio [95% CI] | 0.398 [0.308, 0.514] | |
| Stratified log-rank p-valuec | <0.0001 | |
| ORR (%) [95% CI]a | 83 [77.1, 88.5] | 50 [42.7, 57.8] |
| Odds ratio [95% CI] | 5.31 [3.08, 8.90] | |
| Stratified CMH test p-valuec | <0.0001 | |
| Complete Response Rate (%) | 65 [57.6, 71.9] | 32 [25.6, 39.8] |
| Partial Response Rate (%) | 18 [13.0, 24.8] | 18 [12.6, 24.3] |
| OSd | ||
| Number of events (%) | 82 (46) | 95 (53) |
| Median OS, months [95% CI]b | NR (28.6, NE) | 31.1 (17.1, NE) |
| Stratified hazard ratio [95% CI] | 0.726 (0.540, 0.977) | |
| Stratified log-rank p-valuec,e | 0.0335 | |
CI, confidence interval; CMH, Cochran-Mantel-Haenszel; EFS, event-free survival; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival.
a Per central assessment performed at the time of primary EFS analysis
b Kaplan-Meier method
c The p values are two-sided. Stratified log-rank test or stratified CMH adjusted for response to first-line therapy (primary refractory versus relapse ≤6 months of first-line therapy versus relapse >6 and ≤12 months of first-line therapy) and second-line age-adjusted International Prognostic Index (0 to 1 versus 2 to 3)
d Per assessment performed at the time of primary analysis of OS (five years from the first subject enrolled)
e p-value is compared with 0.0482, the two-sided efficacy boundary (significance level) for the primary OS analysis
Figure 1. Kaplan-Meier Plot of Event-Free Survival in ZUMA-7:
CI, confidence interval; HR, hazard ratio; SOCT, standard of care treatment.
Figure 2. Kaplan-Meier Plot of Overall Survival in ZUMA-7:
CI, confidence interval; HR, hazard ratio; NE, not estimable; SOCT, standard of care treatment.
Note: Subjects who did not respond to SOCT could receive subsequent treatment for lymphoma including anti-CD19 CAR T-cell therapy outside the requirement of the protocol.
Figure 3. Forest Plot of Event-Free Survival in Selected Subgroups in ZUMA-7:
The OS benefit with Yescarta is consistent across clinically relevant subgroups.
Relapsed or refractory FL after three or more lines of systemic therapy (ZUMA-5)
The efficacy and safety of Yescarta in adult patients with FL, were evaluated in a phase 2 single-arm, open-label, multicentre study in patients with r/r FL based on 2016 WHO-classification.
Eligible patients were ≥18 years of age with refractory disease after 2 or more prior lines of therapy. Prior therapy must have included an anti-CD20 monoclonal antibody combined with an alkylating agent (single-agent anti-CD20 antibody did not count as line of therapy for eligibility). Patients with SD (without relapse) >1 year from completion of last therapy were not considered eligible. Patients with CNS lymphoma, a history of allogeneic SCT or prior anti-CD19 CAR or other genetically modified T-cell therapy were excluded. Patients with a history of CNS disorders (such as seizures or cerebrovascular ischemia), left ventricular ejection fraction of less than 50% or room air oxygen saturation of less than 92%, or autoimmune disease requiring systemic immunosuppression were ineligible. The study excluded patients with active or serious infections and patients with FL Grade 3b. The actual duration of follow-up was 25.9 months (range: 0.3 to 44.3 months, still ongoing). A summary of the patient demographics is provided in Table 7.
At the time of the primary analysis, a total of 122 FL patients were enrolled (i.e. leukapheresed), including 75 patients who had received 3 or more lines of previous therapy. In the period between the primary analysis data cut-off date and the 24-month follow-up analysis data cut-off date, no additional patients with FL were enrolled or treated with Yescarta.
Table 7. Summary of demographics for ZUMA-5 FL patients (24-month analysis):
| Category | All leukapheresed (N=122) | All leukapheresed with ≥3 lines of therapy (N=75*) |
|---|---|---|
| Age (years) | ||
| Median (min, max) | 60 (34, 79) | 60 (34, 79) |
| ≥65 | 30% | 31% |
| Male gender | 60% | 63% |
| Race | ||
| White | 93% | 93% |
| Asian | 2% | 4% |
| Black | 2% | 1% |
| ECOG status | ||
| 0 | 63% | 59% |
| 1 | 37% | 41% |
| High tumour bulk as defined by GELF criteria | 52% | 57% |
| Median number of prior therapies (min, max) | 3 (1, 10) | 4 (3, 10) |
| Patients with refractory disease to ≥2 prior lines of therapy | 30% | 24% |
| Patients with disease stage III/IV | 86% | 86% |
| Patients with prior autologous stem cell transplant | 25% | 29% |
| Prior PI3K inhibitor | 26% | 40% |
| Time to relapse from first anti-CD20 chemotherapy combination therapy <24 months | 54% | 51% |
ECOG, Eastern Cooperative Oncology Group; GELF, Groupe d’Etude des Lymphomes Folliculaires.
* All patients with locally confirmed diagnosis, including 60 patients with centralised confirmed diagnosis. Number of leukapheresed (n=75) and treated (n=73) patients.
Yescarta was administered as a single intravenous infusion at a target dose of 2 × 106 anti-CD19 CAR T cells/kg after lymphodepleting chemotherapy regimen of cyclophosphamide 500 mg/m² intravenously and fludarabine 30 mg/m² intravenously, both given on the 5th , 4th , and 3 rd day before Yescarta. All patients were hospitalized for observation for a minimum of 7 days after Yescarta infusion. The administration and monitoring of Yescarta is consistent between ZUMA-5 and ZUMA-1.
The primary analysis was performed, when at least 80 consecutively enrolled FL patients had a minimum follow-up of 12 months from first response assessment. The primary endpoint was ORR. Secondary endpoints included CR rate, ORR and CR in patients who received 3 or more lines of prior therapy, DOR, OS and PFS and incidence of adverse events. Three out of the 122 FL patients enrolled at the time of the primary analysis were not treated, primarily due to ineligibility, experiencing CR prior or death prior to the treatment.
A 24-month follow-up analysis was performed, when at least 80 FL patients had a minimum follow-up of 24 months after infusion.
As of the 24-month follow-up analysis, no additional patients underwent leukapheresis nor were treated with Yescarta. No manufacturing failures occurred. The median time from leukapheresis to product release was 12 days (range: 10 to 37 days), leukapheresis to product delivery was 17 days (range: 13 to 72 days) and leukapheresis to Yescarta infusion was 27 days (range: 19 to 330 days). The median dose was 2.0 × 106 anti-CD19 CAR T cells/kg. At the time of the primary analysis data cut, 122 FL patients were enrolled. Among the 75 enrolled FL patients who had 3 or more lines of prior therapy, the ORR was 91% and the CR rate was 77%.
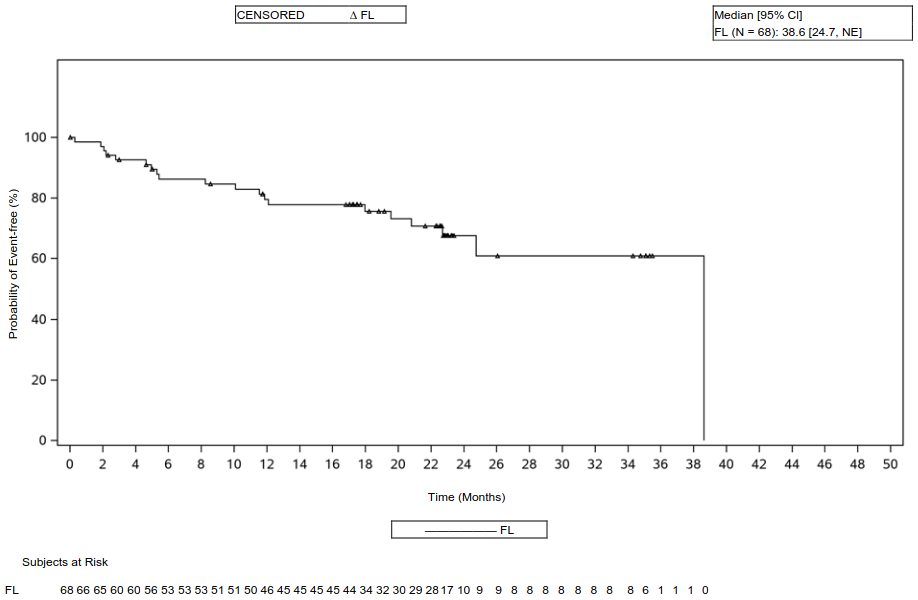
The 24-month follow-up analysis was performed on the 122 enrolled FL patients, and 119 of these patients were treated with Yescarta. Among the 122 enrolled FL patients, 75 had 3 or more lines of prior therapy, resulting in an ORR of 91% and CR rate of 77%. The median time to response was 1 month (range: 0.8 to 3.1 months), the median DOR was 38.6 months and the proportion of responders who remained in response was 62% at Month 24. Twenty nine out of 75 FL patients who had 3 or more prior lines of therapy initially achieved a PR, 19 of whom later achieved CR. Subgroup analysis included ORR in patients who were refractory (88%), FLIPI score ≥3 (94%), high tumour burden (91%), progression of disease within 24 months of first immunotherapy (89%) and prior treatment with PI3K inhibitor (90%). Key efficacy results for FL patients with 3 or more prior lines of therapy are summarized in Table 8.
Table 8. Summary of Efficacy Results for all enrolled ZUMA-5 FL patients with 3 or more prior lines of therapy (24-month analysis):
| Category | All leukapheresed (ITT) N=75* |
|---|---|
| ORRa, (%) [95% CI] | 91% (82, 96) |
| CR, (%) | 77% |
| PR, (%) | 13% |
| DORb, median in months [95% CI] (range) | 38.6 (24.7, NE) (0.0, 38.6) |
| Ongoing Response (n) | 42 |
| Rate of Continued Remissionb % [95% CI] 12 Month 18 Month 24 Month | 79.5(67.2, 87.6) 75.5 (62.5, 84.6) 67.6 (52.7, 78.7) |
CI, confidence interval; CR, complete response; DOR, duration of response; ITT, intention-to-treat; NE, not estimable; ORR, objective response; PR, partial response
a Per the International Working Group Lugano Classification (Cheson 2014), as assessed by the Independent Radiology Review Committee
b Measured from the date of first objective response to the date of progression or death
* All patients with locally confirmed diagnosis, including 60 patients with centralized confirmed diagnosis. Number of leukapheresed (n=75) and treated (n=73) patients.
Figure 4. Kaplan Meier DOR in the all leukapheresed set, patients with objective response (FL patients with 3 or more lines of prior therapy, 24-month analysis, independent review committee):
CI, confidence interval; NE, not estimable.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Yescarta in all subsets of the paediatric population in the treatment of mature B-cell neoplasms (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Yescarta comprises human autologous T cells. The anticipated residual products are typical cellular degradation products resulting from normal cellular clearance mechanisms. Thus, the infused CAR T cells are expected to be cleared over time.
Cellular kinetics
Following infusion of Yescarta anti-CD19 CAR T cells exhibited an initial rapid expansion followed by a decline to near baseline levels by 3 months. Peak levels of anti-CD19 CAR T cells occurred within the first 7 to 14 days after the day of Yescarta infusion. Age (range: 21 to 80 years) and sex had no significant impact on AUC and peak levels of Yescarta.
Among patients in ZUMA-1, the median peak level of anti-CD19 CAR T cells in the blood was 38.3 cells/μL (range: 0.8 to 1513.7 cells/μL), which decreased to a median of 2.1 cells/μL by 1 month (range: 0 to 167.4 cells/μL) and to a median of 0.4 cells/μL by 3 months (range: 0 to 28.4 cells/μL) after Yescarta infusion. Among patients in ZUMA-7 the median peak level of anti-CD19 CAR T cells in the blood was 25.84 cells/μL (range: 0.04 to 1173.25 cells/μL), which decreased towards baseline in evaluable patients by 3 months (0.35 cells/μL; range: 0.00 to 28.44 cells/μL), but were still detectable in 12 out of 30 evaluable patients until 24 months post-treatment.
Among patients in ZUMA-5 with FL, the median peak level of anti-CD19 CAR T cells in the blood was 37.6 cells/μL (range: 0.5 to 1415.4 cells/μL). The median time to peak of anti-CD19 CAR T cells in the blood was 8 days after infusion (range: 8 to 371 days). By 3 months, anti-CD19 CAR T cell levels decreased to near baseline levels to a median of 0.3 cells/μL (range: 0 to 15.8 cells/μL).
Among patients in ZUMA-1, the number of anti-CD19 CAR T cells in the blood was positively associated with objective response (CR or PR). The median anti-CD19 CAR T cell peak level in responders (N=71) was 216% higher compared to the corresponding level in nonresponders (N=25) (43.6 cells/μL versus 20.2 cells/μL). Median AUC0-28 in responding patients (N=71) was 253% of the corresponding level in nonresponders (N=25) (562 days × cells/μL versus 222 days × cells/μL).
Among patients in ZUMA-7 the number of anti-CD19 CAR T cells in the blood was positively associated with objective response (CR or PR). The median anti-CD19 CAR T cell peak levels in responders (n=142) were about 275% higher compared to the corresponding level in nonresponders (n=20) (28.9 cells/μL versus 10.5 cells/μL). Median AUC0-28 in responding patients (n=142) was about 417% higher compared to the corresponding level in nonresponders (n=20) (292.9 days × cells/μL versus 70.1 days × cells/μL).
Among patients with FL in ZUMA-5, the median peak anti-CD19 CAR T-cell levels in responders (n=112) versus nonresponders (n=5) were 38.0 cells/μL and 31.3 cells/μL, respectively. The median AUC0-28 in responders versus nonresponders were 454.8 cells/μL•days and 247.1 cells/μL•days, respectively.
Studies of Yescarta in patients with hepatic and renal impairment were not conducted.
Preclinical safety data
Yescarta comprises engineered human T cells, therefore there are no representative in vitro assays, ex vivo models, or in vivo models that can accurately address the toxicological characteristics of the human product. Hence, traditional toxicology studies used for drug development were not performed.
No carcinogenicity or genotoxicity studies have been conducted with Yescarta.
No studies have been conducted to evaluate the effects of Yescarta on fertility, reproduction, and development.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.